Study of efficacy and antibody duration to fourth-dose booster of Ad5-nCoV or inactivated SARS-CoV-2 vaccine in Chinese adults: a prospective cohort study
- PMID: 37736100
- PMCID: PMC10510200
- DOI: 10.3389/fimmu.2023.1244373
Study of efficacy and antibody duration to fourth-dose booster of Ad5-nCoV or inactivated SARS-CoV-2 vaccine in Chinese adults: a prospective cohort study
Abstract
Introduction: China experienced a record surge of coronavirus disease 2019 cases in December 2022, during the pandemic.
Methods: We conducted a randomized, parallel-controlled prospective cohort study to evaluate efficacy and antibody duration after a fourth-dose booster with Ad5-nCoV or inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine.
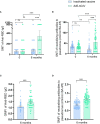
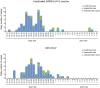
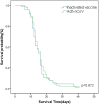
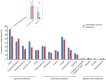
Results: A total of 191 participants aged ≥18 years who had completed a three-dose regimen of the inactivated SARS-CoV-2 vaccine 6 months earlier were recruited to receive the intramuscular Ad5-nCoV booster or the inactivated SARS-CoV-2 vaccine. The Ad5-nCoV group had significantly higher antibody levels compared with the inactivated vaccine group at 6 months after the fourth vaccination dose. After the pandemic, the breakthrough infection rate for the Ad5-nCoV and the inactivated vaccine groups was 77.89% and 78.13%, respectively. Survival curve analysis (p = 0.872) and multivariable logistic regression analysis (p = 0.956) showed no statistically significant differences in breakthrough infection between the two groups.
Discussion: Compared with a homologous fourth dose, a heterologous fourth dose of Ad5-nCoV elicited a higher immunogenic response in healthy adults who had been immunized with three doses of inactivated vaccine. Nevertheless, the efficacy of the two vaccine types was equivalent after the pandemic.
Keywords: Ad5-nCoV; COVID-19; SARS-CoV-2; breakthrough infection; fourth dose.
Copyright © 2023 Xu, Xu, Dai, Zheng, Qin, Wan, Yang, Jiang, Zhang, Hu and Lv.
Conflict of interest statement
YX and PW are employees of CanSino Biologics and contributed to the conceptualization of the study clinical protocol and electronic case report form design but did not participate in the analysis or interpretation of the data presented in the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety and immunogenicity of Ad5-nCoV immunization after three-dose priming with inactivated SARS-CoV-2 vaccine in Chinese adults.Nat Commun. 2023 Aug 8;14(1):4757. doi: 10.1038/s41467-023-40489-2. Nat Commun. 2023. PMID: 37553338 Free PMC article. Clinical Trial.
-
Immune responses and transcription landscape of adults with the third dose of homologous and heterologous booster vaccines of COVID-19.Front Immunol. 2024 Sep 12;15:1461419. doi: 10.3389/fimmu.2024.1461419. eCollection 2024. Front Immunol. 2024. PMID: 39328415 Free PMC article.
-
Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial.Lancet Respir Med. 2023 Jul;11(7):613-623. doi: 10.1016/S2213-2600(23)00049-8. Epub 2023 Mar 7. Lancet Respir Med. 2023. PMID: 36898400 Free PMC article. Clinical Trial.
-
A China-developed adenovirus vector-based COVID-19 vaccine: review of the development and application of Ad5-nCov.Expert Rev Vaccines. 2023 Jan-Dec;22(1):704-713. doi: 10.1080/14760584.2023.2242528. Expert Rev Vaccines. 2023. PMID: 37501516 Review.
-
Anti-SARS-CoV-2 spike IgG following injection of the third dose vaccine: A systematic review with meta-analysis of heterologous versus homologous vaccination.Front Public Health. 2023 Jan 12;10:960598. doi: 10.3389/fpubh.2022.960598. eCollection 2022. Front Public Health. 2023. PMID: 36711369 Free PMC article.
Cited by
-
Comparable immune escape capacity between KP.2 and other SARS-CoV-2 variants in the central Chinese population after the first COVID-19 booster.Sci Rep. 2025 May 22;15(1):17762. doi: 10.1038/s41598-025-02927-7. Sci Rep. 2025. PMID: 40404955 Free PMC article.
References
-
- Statement on the second meeting of the international health regulations (2005) emergency committee regarding the outbreak of novel coronavirus (2019-nCoV). (2020). Available at: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting....
-
- Organization WH . (2023). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Organization WH . Statement on the fourteenth meeting of the international health regulations (2005) emergency committee regarding the coronavirus disease (COVID-19) pandemic. (2023). Available at: https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-mee....
-
- Arashiro T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y, et al. . Covid-19 vaccine effectiveness against symptomatic sars-Cov-2 infection during delta-dominant and omicron-dominant periods in japan: A multi-center prospective case-control study (Fascinate study). Clin Infect Dis (2022) 76(3):e108–15. doi: 10.1093/cid/ciac635 - DOI - PMC - PubMed
-
- Gram MA, Emborg HD, Schelde AB, Friis NU, Nielsen KF, Moustsen-Helms IR, et al. . Vaccine effectiveness against sars-Cov-2 infection or covid-19 hospitalization with the alpha, delta, or omicron sars-Cov-2 variant: A nationwide danish cohort study. PloS Med (2022) 19(9):e1003992. doi: 10.1371/journal.pmed.1003992 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

