The multifaceted role of macrophages during acute liver injury
- PMID: 37736102
- PMCID: PMC10510203
- DOI: 10.3389/fimmu.2023.1237042
The multifaceted role of macrophages during acute liver injury
Abstract
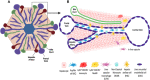
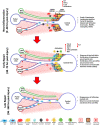
The liver is situated at the interface of the gut and circulation where it acts as a filter for blood-borne and gut-derived microbes and biological molecules, promoting tolerance of non-invasive antigens while driving immune responses against pathogenic ones. Liver resident immune cells such as Kupffer cells (KCs), a subset of macrophages, maintain homeostasis under physiological conditions. However, upon liver injury, these cells and others recruited from circulation participate in the response to injury and the repair of tissue damage. Such response is thus spatially and temporally regulated and implicates interconnected cells of immune and non-immune nature. This review will describe the hepatic immune environment during acute liver injury and the subsequent wound healing process. In its early stages, the wound healing immune response involves a necroinflammatory process characterized by partial depletion of resident KCs and lymphocytes and a significant infiltration of myeloid cells including monocyte-derived macrophages (MoMFs) complemented by a wave of pro-inflammatory mediators. The subsequent repair stage includes restoring KCs, initiating angiogenesis, renewing extracellular matrix and enhancing proliferation/activation of resident parenchymal and mesenchymal cells. This review will focus on the multifaceted role of hepatic macrophages, including KCs and MoMFs, and their spatial distribution and roles during acute liver injury.
Keywords: acute injury; liver; macrophages; necroinflammation; spatial distribution; tissue repair; wound healing response.
Copyright © 2023 Hassan, Flores Molina and Shoukry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Distinct spatial distribution and roles of Kupffer cells and monocyte-derived macrophages in mouse acute liver injury.Front Immunol. 2022 Sep 30;13:994480. doi: 10.3389/fimmu.2022.994480. eCollection 2022. Front Immunol. 2022. PMID: 36248843 Free PMC article.
-
Characterisation of macrophages in healthy and diseased livers in mice: identification of necrotic lesion-associated macrophages.eGastroenterology. 2025 Apr 7;3(2):e100189. doi: 10.1136/egastro-2025-100189. eCollection 2025. eGastroenterology. 2025. PMID: 40212045 Free PMC article.
-
The versatility of macrophage heterogeneity in liver fibrosis.Front Immunol. 2022 Aug 5;13:968879. doi: 10.3389/fimmu.2022.968879. eCollection 2022. Front Immunol. 2022. PMID: 35990625 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury.J Immunol. 2017 May 1;198(9):3588-3595. doi: 10.4049/jimmunol.1601428. Epub 2017 Mar 13. J Immunol. 2017. PMID: 28289160 Free PMC article.
Cited by
-
Macrophage PKM2 depletion ameliorates hepatic inflammation and acute liver injury in mice.Front Pharmacol. 2025 Apr 25;16:1546045. doi: 10.3389/fphar.2025.1546045. eCollection 2025. Front Pharmacol. 2025. PMID: 40351417 Free PMC article.
-
Zebrafish as a model for human epithelial pathology.Lab Anim Res. 2025 Feb 3;41(1):6. doi: 10.1186/s42826-025-00238-6. Lab Anim Res. 2025. PMID: 39901304 Free PMC article. Review.
-
CX3CL1 (Fractalkine)-CX3CR1 Axis in Inflammation-Induced Angiogenesis and Tumorigenesis.Int J Mol Sci. 2024 Apr 25;25(9):4679. doi: 10.3390/ijms25094679. Int J Mol Sci. 2024. PMID: 38731899 Free PMC article. Review.
-
Liver macrophages revisited: The expanding universe of versatile responses in a spatiotemporal context.Hepatol Commun. 2024 Jul 5;8(7):e0491. doi: 10.1097/HC9.0000000000000491. eCollection 2024 Jul 1. Hepatol Commun. 2024. PMID: 38967563 Free PMC article. Review.
-
The efferocytosis process in aging: Supporting evidence, mechanisms, and therapeutic prospects for age-related diseases.J Adv Res. 2025 Mar;69:31-49. doi: 10.1016/j.jare.2024.03.008. Epub 2024 Mar 17. J Adv Res. 2025. PMID: 38499245 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

