Discovery of Novel, Thienopyridine-Based Tyrosine Kinase Inhibitors Targeting Tumorigenic RON Splice Variants
- PMID: 37736180
- PMCID: PMC10510527
- DOI: 10.1021/acsmedchemlett.3c00206
Discovery of Novel, Thienopyridine-Based Tyrosine Kinase Inhibitors Targeting Tumorigenic RON Splice Variants
Abstract
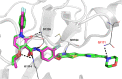
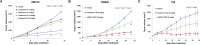
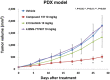
Herein, we report the identification, structural optimization, and biological efficacy of thieno[2,3-b]pyridines as potent inhibitors of splice variants of the tyrosine kinase recepteur d'origine nantais (RON). Among synthesized compounds, compound 15f exhibited excellent in vitro kinase inhibition and antiproliferative activity, as well as in vivo antineoplastic efficacy against RON splice variant-expressing tumors. Moreover, compound 15f with excellent pharmacokinetics demonstrated significant activity with greater tumor growth inhibition (74.9% at 10 mg/kg) than compounds 2 and 4 in a patient-derived xenograft model. Collectively, 15f represents a promising, novel anticancer agent targeting RON splice variants.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




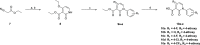


References
LinkOut - more resources
Full Text Sources
Chemical Information

