Safety and efficacy of a novel 3D-printed bioresorbable sirolimus-eluting scaffold in a porcine model
- PMID: 37736208
- PMCID: PMC10507451
- DOI: 10.4244/AIJ-D-22-00051
Safety and efficacy of a novel 3D-printed bioresorbable sirolimus-eluting scaffold in a porcine model
Abstract
Background: The effect of 3D-printed bioresorbable vascular scaffolds (BRS) in coronary heart disease has not been clarified.
Aims: We aimed to compare the safety and efficacy of 3D-printed BRS with that of metallic sirolimus-eluting stents (SES).
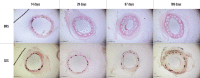
Methods: Thirty-two BRS and 32 SES were implanted into 64 porcine coronary arteries. Quantitative coronary angiography (QCA) and optical coherence tomography (OCT) were performed at 14, 28, 97, and 189 days post-implantation. Scanning electron microscopy (SEM) and histopathological analyses were performed at each assessment.
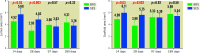
Results: All stents/scaffolds were successfully implanted. All animals survived for the duration of the study. QCA showed the two devices had a similar stent/scaffold-to-artery ratio and acute percent recoil. OCT showed the lumen area (LA) and scaffold/stent area (SA) of the BRS were significantly smaller than those of the SES at 14 and 28 days post-implantation (14-day LA: BRS vs SES 4.52±0.41 mm2 vs 5.69±1.11 mm2; p=0.03; 14-day SA: BRS vs SES 4.99±0.45 mm2 vs 6.11±1.06 mm2; p=0.03; 28-day LA: BRS vs SES 2.93±1.03 mm2 vs 4.82±0.74 mm2; p=0.003; 28-day SA: BRS vs SES 3.86±0.98 mm2 vs 5.75±0.71 mm2; p=0.03). Both the LA and SA of the BRS increased over time and were similar to those of the SES at the 97-day and 189-day assessments. SEM and histomorphological analyses showed no significant between-group differences in endothelialisation at each assessment.
Conclusions: The novel 3D-printed BRS showed safety and efficacy similar to that of SES in a porcine model. The BRS also showed a long-term positive remodelling effect.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Ormiston JA, Serruys PW. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009;2:255–60. - PubMed
-
- Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur Heart J. 2012;33:16–25b. - PubMed
-
- Garcia-Garcia HM, Serruys PW, Campos CM, Muramatsu T, Nakatani S, Zhang YJ, Onuma Y, Stone GW. Assessing bioresorbable coronary devices: methods and parameters. JACC Cardiovasc Imaging. 2014;7:1130–48. - PubMed
-
- Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW ABSORB III Investigators. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J Am Coll Cardiol. 2017;70:2852–62. - PubMed
-
- Elias J, van Dongen, Kraak RP, Tijssen RYG, Claessen BEPM, Tijssen JGP, de Winter, Piek JJ, Wykrzykowska JJ, Henriques JPS. Mid-term and long-term safety and efficacy of bioresorbable vascular scaffolds versus metallic everolimus-eluting stents in coronary artery disease: A weighted meta-analysis of seven randomised controlled trials including 5577 patients. Neth Heart J. 2017;25:429–38. - PMC - PubMed
LinkOut - more resources
Full Text Sources

