In situ forming biomaterials as muscle void fillers for the provisional treatment of volumetric muscle loss injuries
- PMID: 37736246
- PMCID: PMC10509707
- DOI: 10.1016/j.mtbio.2023.100781
In situ forming biomaterials as muscle void fillers for the provisional treatment of volumetric muscle loss injuries
Abstract
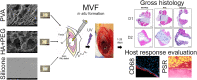
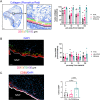
Volumetric muscle loss (VML) represents a devastating extremity injury which leads to chronic functional deficits and disability and is unrecoverable through normal healing pathways. When left untreated, the VML pathophysiology creates many challenges towards successful treatment, such as altered residual muscle architecture, excessive fibrosis, and contracture(s). As such, innovative approaches and technologies are needed to prevent or reverse these adverse sequelae. Development of a rationally designed biomaterial technology which is intended to be acutely placed within a VML defect - i.e., to serve as a muscle void filler (MVF) by maintaining the VML defect - could address this clinical unmet need by preventing these adverse sequelae as well as enabling multi-staged treatment approaches. To that end, three biomaterials were evaluated for their ability to serve as a provisional MVF treatment intended to stabilize a VML defect in a rat model for an extended period (28 days): polyvinyl alcohol (PVA), hyaluronic acid and polyethylene glycol combination (HA + PEG), and silicone, a clinically used soft tissue void filler. HA + PEG biomaterial showed signs of deformation, while both PVA and silicone did not. There were no differences between treatment groups for their effects on adjacent muscle fiber count and size distribution. Not surprisingly, silicone elicited robust fibrotic response resulting in a fibrotic barrier with a large infiltration of macrophages, a response not seen with either the PVA or HA + PEG. Taken together, PVA was found to be the best material to be used as a provisional MVF for maintaining VML defect volume while minimizing adverse effects on the surrounding muscle.
Keywords: Biocompatibility; Extremities; Military medicine; Prolonged care; Trauma.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Christopher L. Dearth, PhD reports financial support was provided by DoD Extremity Trauma & Amputation Center of Excellence. Christopher L. Dearth, PhD reports financial support was provided by US Army Medical Research and Development Command. Christopher L. Dearth, PhD reports financial support was provided by Orthopaedic Trauma Association.
Figures



Similar articles
-
A Two-Stage Approach Integrating Provisional Biomaterial-Mediated Stabilization Followed by a Definitive Treatment for Managing Volumetric Muscle Loss Injuries.J Funct Biomater. 2024 Jun 6;15(6):160. doi: 10.3390/jfb15060160. J Funct Biomater. 2024. PMID: 38921533 Free PMC article.
-
Evaluating the potential use of functional fibrosis to facilitate improved outcomes following volumetric muscle loss injury.Acta Biomater. 2022 Mar 1;140:379-388. doi: 10.1016/j.actbio.2021.11.032. Epub 2021 Nov 26. Acta Biomater. 2022. PMID: 34843950
-
Acrylated Hyaluronic-Acid Based Hydrogel for the Treatment of Craniofacial Volumetric Muscle Loss.Tissue Eng Part A. 2024 Nov;30(21-22):704-711. doi: 10.1089/ten.TEA.2023.0241. Epub 2024 Apr 9. Tissue Eng Part A. 2024. PMID: 38534963
-
Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss.Bioengineering (Basel). 2020 Jul 31;7(3):85. doi: 10.3390/bioengineering7030085. Bioengineering (Basel). 2020. PMID: 32751847 Free PMC article. Review.
-
Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss.Cells. 2021 Aug 7;10(8):2016. doi: 10.3390/cells10082016. Cells. 2021. PMID: 34440785 Free PMC article. Review.
Cited by
-
A Two-Stage Approach Integrating Provisional Biomaterial-Mediated Stabilization Followed by a Definitive Treatment for Managing Volumetric Muscle Loss Injuries.J Funct Biomater. 2024 Jun 6;15(6):160. doi: 10.3390/jfb15060160. J Funct Biomater. 2024. PMID: 38921533 Free PMC article.
-
In-vivo evaluation of vitamin E loaded muscle void fillers for the provisional treatment of volumetric muscle loss.BMC Res Notes. 2025 Aug 29;18(1):372. doi: 10.1186/s13104-025-07454-2. BMC Res Notes. 2025. PMID: 40883770 Free PMC article.
References
-
- Masquelet A.C., Fitoussi F., Begue T., Muller G.P. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann. Chir. Plast. Esthet. 2000;45(3):346–353. - PubMed
-
- Dirschl D.R., Del Gaizo D. Staged management of tibial plateau fractures. Am. J. Orthoped. 2007;36(4 Suppl):12–17. - PubMed
-
- Keenan S., Riesberg J.C. Prolonged field care: beyond the "golden hour". Wilderness Environ. Med. 2017;28(2S):S135–S139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

