A Rab-bit hole: Rab40 GTPases as new regulators of the actin cytoskeleton and cell migration
- PMID: 37736498
- PMCID: PMC10509765
- DOI: 10.3389/fcell.2023.1268922
A Rab-bit hole: Rab40 GTPases as new regulators of the actin cytoskeleton and cell migration
Abstract
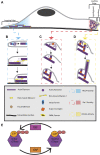
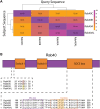
The regulation of machinery involved in cell migration is vital to the maintenance of proper organism function. When migration is dysregulated, a variety of phenotypes ranging from developmental disorders to cancer metastasis can occur. One of the primary structures involved in cell migration is the actin cytoskeleton. Actin assembly and disassembly form a variety of dynamic structures which provide the pushing and contractile forces necessary for cells to properly migrate. As such, actin dynamics are tightly regulated. Classically, the Rho family of GTPases are considered the major regulators of the actin cytoskeleton during cell migration. Together, this family establishes polarity in the migrating cell by stimulating the formation of various actin structures in specific cellular locations. However, while the Rho GTPases are acknowledged as the core machinery regulating actin dynamics and cell migration, a variety of other proteins have become established as modulators of actin structures and cell migration. One such group of proteins is the Rab40 family of GTPases, an evolutionarily and functionally unique family of Rabs. Rab40 originated as a single protein in the bilaterians and, through multiple duplication events, expanded to a four-protein family in higher primates. Furthermore, unlike other members of the Rab family, Rab40 proteins contain a C-terminally located suppressor of cytokine signaling (SOCS) box domain. Through the SOCS box, Rab40 proteins interact with Cullin5 to form an E3 ubiquitin ligase complex. As a member of this complex, Rab40 ubiquitinates its effectors, controlling their degradation, localization, and activation. Because substrates of the Rab40/Cullin5 complex can play a role in regulating actin structures and cell migration, the Rab40 family of proteins has recently emerged as unique modulators of cell migration machinery.
Keywords: Rab40 GTPases; Rho GTPases; SOCS box; actin; cell migration; cytoskeleton; ubiquitination.
Copyright © 2023 Neumann and Prekeris.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

