Why surface hydrophobicity promotes CO2 electroreduction: a case study of hydrophobic polymer N-heterocyclic carbenes
- PMID: 37736633
- PMCID: PMC10510627
- DOI: 10.1039/d3sc02658b
Why surface hydrophobicity promotes CO2 electroreduction: a case study of hydrophobic polymer N-heterocyclic carbenes
Abstract
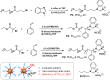
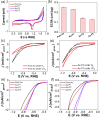
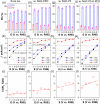
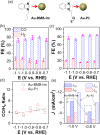
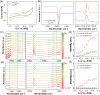
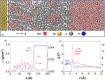
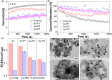
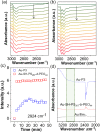
We report the use of polymer N-heterocyclic carbenes (NHCs) to control the microenvironment surrounding metal nanocatalysts, thereby enhancing their catalytic performance in CO2 electroreduction. Three polymer NHC ligands were designed with different hydrophobicity: hydrophilic poly(ethylene oxide) (PEO-NHC), hydrophobic polystyrene (PS-NHC), and amphiphilic block copolymer (BCP) (PEO-b-PS-NHC). All three polymer NHCs exhibited enhanced reactivity of gold nanoparticles (AuNPs) during CO2 electroreduction by suppressing proton reduction. Notably, the incorporation of hydrophobic PS segments in both PS-NHC and PEO-b-PS-NHC led to a twofold increase in the partial current density for CO formation, as compared to the hydrophilic PEO-NHC. While polymer ligands did not hinder ion diffusion, their hydrophobicity altered the localized hydrogen bonding structures of water. This was confirmed experimentally and theoretically through attenuated total reflectance surface-enhanced infrared absorption spectroscopy and molecular dynamics simulation, demonstrating improved CO2 diffusion and subsequent reduction in the presence of hydrophobic polymers. Furthermore, NHCs exhibited reasonable stability under reductive conditions, preserving the structural integrity of AuNPs, unlike thiol-ended polymers. The combination of NHC binding motifs with hydrophobic polymers provides valuable insights into controlling the microenvironment of metal nanocatalysts, offering a bioinspired strategy for the design of artificial metalloenzymes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Gold Nanoparticles Grafted with Molecular N-Heterocyclic Carbenes for CO2 Electroreduction: Combining Electronic and Hydrophobic Effects.ACS Appl Mater Interfaces. 2025 Jul 30;17(30):43899-43908. doi: 10.1021/acsami.5c09618. Epub 2025 Jul 16. ACS Appl Mater Interfaces. 2025. PMID: 40668760
-
A Polymer Solution To Prevent Nanoclustering and Improve the Selectivity of Metal Nanoparticles for Electrocatalytic CO2 Reduction.Angew Chem Int Ed Engl. 2019 Oct 28;58(44):15834-15840. doi: 10.1002/anie.201909069. Epub 2019 Sep 20. Angew Chem Int Ed Engl. 2019. PMID: 31468668 Review.
-
Polymer N-Heterocyclic Carbene (NHC) Ligands for Silver Nanoparticles.ACS Appl Mater Interfaces. 2022 Dec 14;14(49):55227-55237. doi: 10.1021/acsami.2c17706. Epub 2022 Dec 2. ACS Appl Mater Interfaces. 2022. PMID: 36459050
-
N-Heterocyclic Carbene Polymer-Stabilized Au Nanowires for Selective and Stable Reduction of CO2.J Am Chem Soc. 2025 Apr 30;147(17):14845-14855. doi: 10.1021/jacs.5c04742. Epub 2025 Apr 16. J Am Chem Soc. 2025. PMID: 40238718
-
N-Heterocyclic carbene-functionalized metal nanoparticles and nanoclusters for nanocatalysis.Dalton Trans. 2024 Nov 26;53(46):18440-18450. doi: 10.1039/d4dt02434f. Dalton Trans. 2024. PMID: 39422710 Review.
Cited by
-
Fluorinated polymer zwitterions on gold nanoparticles: patterned catalyst surfaces guide interfacial transport and electrochemical CO2 reduction.Nanoscale. 2024 Aug 22;16(33):15558-15567. doi: 10.1039/d4nr01484g. Nanoscale. 2024. PMID: 39101249 Free PMC article.
-
Forming N-heterocyclic carbene monolayers: not all deposition methods are the same.Nanoscale. 2025 Feb 27;17(9):5413-5428. doi: 10.1039/d4nr04428b. Nanoscale. 2025. PMID: 39895613 Free PMC article.
-
One-step functionalization of gold nanorods with N-heterocyclic carbene ligands.RSC Adv. 2025 Feb 14;15(7):5007-5010. doi: 10.1039/d5ra00754b. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39957825 Free PMC article.
References
LinkOut - more resources
Full Text Sources

