Stabilisation of the RirA [4Fe-4S] cluster results in loss of iron-sensing function
- PMID: 37736639
- PMCID: PMC10510648
- DOI: 10.1039/d3sc03020b
Stabilisation of the RirA [4Fe-4S] cluster results in loss of iron-sensing function
Abstract
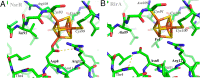
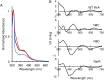
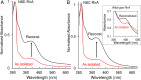
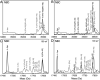
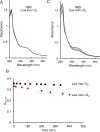
RirA is a global iron regulator in diverse Alphaproteobacteria that belongs to the Rrf2 superfamily of transcriptional regulators, which can contain an iron-sulfur (Fe-S) cluster. Under iron-replete conditions, RirA contains a [4Fe-4S] cluster, enabling high-affinity binding to RirA-regulated operator sequences, thereby causing the repression of cellular iron uptake. Under iron deficiency, one of the cluster irons dissociates, generating an unstable [3Fe-4S] form that subsequently degrades to a [2Fe-2S] form and then to apo RirA, resulting in loss of high-affinity DNA-binding. The cluster is coordinated by three conserved cysteine residues and an unknown fourth ligand. Considering the lability of one of the irons and the resulting cluster fragility, we hypothesized that the fourth ligand may not be an amino acid residue. To investigate this, we considered that the introduction of an amino acid residue that could coordinate the cluster might stabilize it. A structural model of RirA, based on the Rrf2 family nitrosative stress response regulator NsrR, highlighted residue 8, an Asn in the RirA sequence, as being appropriately positioned to coordinate the cluster. Substitution of Asn8 with Asp, the equivalent, cluster-coordinating residue of NsrR, or with Cys, resulted in proteins that contained a [4Fe-4S] cluster, with N8D RirA exhibiting spectroscopic properties very similar to NsrR. The variant proteins retained the ability to bind RirA-regulated DNA, and could still act as repressors of RirA-regulated genes in vivo. However, they were significantly more stable than wild-type RirA when exposed to O2 and/or low iron. Importantly, they exhibited reduced capacity to respond to cellular iron levels, even abolished in the case of the N8D version, and thus were no longer iron sensing. This work demonstrates the importance of cluster fragility for the iron-sensing function of RirA, and more broadly, how a single residue substitution can alter cluster coordination and functional properties in the Rrf2 superfamily of regulators.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflicts to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

