Direct decarboxylative Giese amidations: photocatalytic vs. metal- and light-free
- PMID: 37736650
- PMCID: PMC10510818
- DOI: 10.1039/d3sc03143h
Direct decarboxylative Giese amidations: photocatalytic vs. metal- and light-free
Abstract
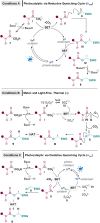
A direct intermolecular decarboxylative Giese amidation reaction from bench stable, non-toxic and environmentally benign oxamic acids has been developed, which allows for easy access to 1,4-difunctionalised compounds which are not otherwise readily accessible. Crucially, a more general acceptor substrate scope is now possible, which renders the Giese amidation applicable to more complex substrates such as natural products and chiral building blocks. Two different photocatalytic methods (one via oxidative and the other via reductive quenching cycles) and one metal- and light-free method were developed and the flexibility provided by different conditions proved to be crucial for enabling a more general substrate scope.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Light-Mediated Direct Decarboxylative Giese Aroylations without a Photocatalyst.J Org Chem. 2024 Nov 1;89(21):16055-16059. doi: 10.1021/acs.joc.4c02163. Epub 2024 Oct 22. J Org Chem. 2024. PMID: 39438444 Free PMC article.
-
Visible Light-Mediated Decarboxylative Giese Addition Utilizing Thioxanthone or Thioxanthone-TfOH Complex as the Photocatalyst.Chemistry. 2025 Feb 25;31(12):e202404483. doi: 10.1002/chem.202404483. Epub 2025 Feb 7. Chemistry. 2025. PMID: 39887856
-
Photoinduced Triphenylphosphine and Iodide Salt Promoted Reductive Decarboxylative Coupling.Adv Sci (Weinh). 2024 Mar;11(12):e2307241. doi: 10.1002/advs.202307241. Epub 2024 Jan 17. Adv Sci (Weinh). 2024. PMID: 38234213 Free PMC article.
-
Synthesis of Alkylated Polyfluorobenzenes through Decarboxylative Giese Addition of Aliphatic N-Hydroxyphthalimide Esters with Polyfluorostyrene.J Org Chem. 2023 Oct 6;88(19):14105-14114. doi: 10.1021/acs.joc.3c01672. Epub 2023 Sep 14. J Org Chem. 2023. PMID: 37708081
-
Direct decarboxylative Giese reactions.Chem Soc Rev. 2022 Feb 21;51(4):1415-1453. doi: 10.1039/d1cs01168e. Chem Soc Rev. 2022. PMID: 35099488 Review.
Cited by
-
Silver-Catalyzed Decarboxylative Coupling of Oxamic Acids with Styrenes to Synthesize E-Cinnamamides: A Distinguish Reaction Pathway.ChemistryOpen. 2025 Aug;14(8):e202400513. doi: 10.1002/open.202400513. Epub 2025 Jan 29. ChemistryOpen. 2025. PMID: 39888278 Free PMC article.
-
Recent Developments in Photoinduced Decarboxylative Acylation of α-Keto Acids.Molecules. 2024 Aug 18;29(16):3904. doi: 10.3390/molecules29163904. Molecules. 2024. PMID: 39202983 Free PMC article. Review.
-
Light-Mediated Direct Decarboxylative Giese Aroylations without a Photocatalyst.J Org Chem. 2024 Nov 1;89(21):16055-16059. doi: 10.1021/acs.joc.4c02163. Epub 2024 Oct 22. J Org Chem. 2024. PMID: 39438444 Free PMC article.
-
Visible light mediated iron-catalyzed addition of oxamic acids to imines.RSC Adv. 2024 Apr 18;14(18):12528-12532. doi: 10.1039/d4ra02258k. eCollection 2024 Apr 16. RSC Adv. 2024. PMID: 38638815 Free PMC article.
-
Visible-Light Photocatalysis for Sustainable Chromene Synthesis and Functionalization.Chemistry. 2025 May 22;31(29):e202500283. doi: 10.1002/chem.202500283. Epub 2025 Apr 3. Chemistry. 2025. PMID: 40100639 Free PMC article. Review.
References
-
- Merkens K. Aguilar Troyano F. J. Anwar K. Gómez-Suárez A. J. Org. Chem. 2021;86:8448–8456. doi: 10.1021/acs.joc.0c02951. - DOI - PubMed
- Zhang O. Schubert J. W. J. Org. Chem. 2020;85:6225–6232. doi: 10.1021/acs.joc.0c00635. - DOI - PubMed
- Ji P. Zhang Y. Dong Y. Huang H. Wei Y. Wang W. Org. Lett. 2020;22:1557–1562. doi: 10.1021/acs.orglett.0c00154. - DOI - PMC - PubMed
- Shah A. A. Kelly, III M. J. Perkins J. J. Org. Lett. 2020;22:2196–2200. doi: 10.1021/acs.orglett.0c00371. - DOI - PubMed
LinkOut - more resources
Full Text Sources

