Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange
- PMID: 37736655
- PMCID: PMC10510812
- DOI: 10.1039/d3sc03078d
Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange
Abstract
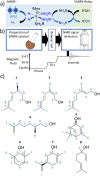
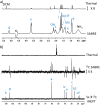
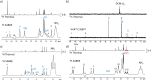
In this work, the limited sensitivity of magnetic resonance is addressed by using the hyperpolarisation method relayed signal amplification by reversible exchange (SABRE-Relay) to transfer latent magnetism from para-hydrogen, a readily isolated spin isomer of hydrogen gas, to components of key plant oils such as citronellol, geraniol, and nerol. This is achieved via relayed polarisation transfer in which an [Ir(H)2(IMes)(NH2R)3]Cl type complex produces hyperpolarised NH2R free in solution, before labile proton exchange between the hyperpolarisation carrier (NH2R) and the OH-containing plant oil component generates enhanced NMR signals for the latter. Consequently, up to ca. 200-fold 1H (0.65% 1H polarisation) and 800-fold 13C NMR signal enhancements (0.65% 13C polarisation) are recorded for these essential oils in seconds. Remarkably, the resulting NMR signals are not only diagnostic, but prove to propagate over large spin systems via a suitable coupling network. A route to optimise the enhancement process by varying the identity of the carrier NH2R, and its concentration is demonstrated. In order to prove utility, these pilot measurements are extended to study a much wider range of plant-derived molecules including rhodinol, verbenol, (1R)-endo-(+)-fenchyl alcohol, (-)-carveol, and linalool. Further measurements are then described which demonstrate citronellol and geraniol can be detected in an off-the-shelf healthcare product rose geranium oil at concentrations of just a few tens of μM in single scan 1H NMR measurements, which are not visible in comparable thermally polarised NMR experiments. This work therefore presents a significant expansion of the types of molecules amenable to hyperpolarisation using para-hydrogen and illustrates a real-world application in the diagnostic detection of low concentration analytes in mixtures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
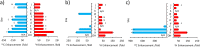

References
-
- Angelovski G. Tickner B. J. Wang G. Nat. Chem. 2023:1–9. - PubMed
LinkOut - more resources
Full Text Sources

