Oral squamous cell carcinomas: state of the field and emerging directions
- PMID: 37736748
- PMCID: PMC10517027
- DOI: 10.1038/s41368-023-00249-w
Oral squamous cell carcinomas: state of the field and emerging directions
Abstract
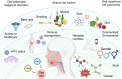
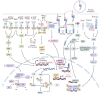
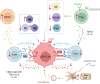
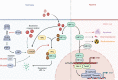
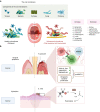
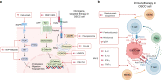
Oral squamous cell carcinoma (OSCC) develops on the mucosal epithelium of the oral cavity. It accounts for approximately 90% of oral malignancies and impairs appearance, pronunciation, swallowing, and flavor perception. In 2020, 377,713 OSCC cases were reported globally. According to the Global Cancer Observatory (GCO), the incidence of OSCC will rise by approximately 40% by 2040, accompanied by a growth in mortality. Persistent exposure to various risk factors, including tobacco, alcohol, betel quid (BQ), and human papillomavirus (HPV), will lead to the development of oral potentially malignant disorders (OPMDs), which are oral mucosal lesions with an increased risk of developing into OSCC. Complex and multifactorial, the oncogenesis process involves genetic alteration, epigenetic modification, and a dysregulated tumor microenvironment. Although various therapeutic interventions, such as chemotherapy, radiation, immunotherapy, and nanomedicine, have been proposed to prevent or treat OSCC and OPMDs, understanding the mechanism of malignancies will facilitate the identification of therapeutic and prognostic factors, thereby improving the efficacy of treatment for OSCC patients. This review summarizes the mechanisms involved in OSCC. Moreover, the current therapeutic interventions and prognostic methods for OSCC and OPMDs are discussed to facilitate comprehension and provide several prospective outlooks for the fields.
© 2023. West China School of Stomatology Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Ng JH, Iyer NG, Tan M-H, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39:297–304. - PubMed
-
- Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–2299. - PubMed
-
- Safi A-F, et al. Clinicopathological parameters affecting nodal yields in patients with oral squamous cell carcinoma receiving selective neck dissection. J. Cranio-Maxillofac. Surg. 2017;45:2092–2096. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

