OXPHOS-targeting drugs in oncology: new perspectives
- PMID: 37736880
- PMCID: PMC11034819
- DOI: 10.1080/14728222.2023.2261631
OXPHOS-targeting drugs in oncology: new perspectives
Abstract
Introduction: Drugs targeting mitochondria are emerging as promising antitumor therapeutics in preclinical models. However, a few of these drugs have shown clinical toxicity. Developing mitochondria-targeted modified natural compounds and US FDA-approved drugs with increased therapeutic index in cancer is discussed as an alternative strategy.
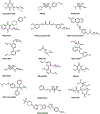
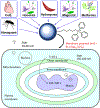
Areas covered: Triphenylphosphonium cation (TPP+)-based drugs selectively accumulate in the mitochondria of cancer cells due to their increased negative membrane potential, target the oxidative phosphorylation proteins, inhibit mitochondrial respiration, and inhibit tumor proliferation. TPP+-based drugs exert minimal toxic side effects in rodents and humans. These drugs can sensitize radiation and immunotherapies.
Expert opinion: TPP+-based drugs targeting the tumor mitochondrial electron transport chain are a new class of oxidative phosphorylation inhibitors with varying antiproliferative and antimetastatic potencies. Some of these TPP+-based agents, which are synthesized from naturally occurring molecules and FDA-approved drugs, have been tested in mice and did not show notable toxicity, including neurotoxicity, when used at doses under the maximally tolerated dose. Thus, more effort should be directed toward the clinical translation of TPP+-based OXPHOS-inhibiting drugs in cancer prevention and treatment.
Keywords: Mitochondrial targets; mitochondrial therapeutics; tumor cells; tumor metastasis; tumor xenografts.
Plain language summary
Mitochondria, which are the cell’s powerhouse of energy, are functional in cancer cells. Inhibition of cancer cell respiration is associated with inhibition of cancer cell proliferation. Therefore, mitochondria have become a promising target for developing antitumor drugs to treat cancer. Several classes of drug molecules selectively target cancer cell mitochondria and inhibit mitochondrial respiration or oxidative phosphorylation (OXPHOS). A new class of OXPHOS-targeting drugs is emerging as a potential cancer therapeutic. One of the OXPHOS inhibitor drugs, IACS-010759, developed by investigators at MD Anderson Cancer Center, was tested in patients with acute myeloid leukemia. Patients who were administered the drug developed peripheral neuropathy and other complications (lactic acidosis), resulting in dose reduction. At lower doses, this drug was not effective. Subsequently, the clinical trial was terminated. The investigators then showed the same type of neurotoxicity using a mouse model. These findings were recently published. Thus, there is an urgent need to develop new OXPHOS inhibitors that do not have neurotoxicity in mice or humans.In this opinion article, we make a case that there are other triphenylphosphonium cation (TPP+)-based mitochondrial OXPHOS inhibitors (inhibiting both complex I and complex III) that are structural modifications of naturally occurring molecules or US FDA-approved drugs. These mitochondria-targeted drugs (MTDs) are as potent as IACS-010759 in cells and in preclinical models. Several TPP+-based MTDs have been tested in mice and did not exert neurotoxicity. TPP+-containing MTDs such as mitochondria-targeted coenzyme Q10 (MitoQ) have been tested in patients with Parkinson’s disease, with no evidence of peripheral neuropathy or other toxicity (e.g., lactic acidosis). Other US FDA-approved drugs (metformin and atovaquone [ATO] or papaverine) are in clinical trials alone or in combination with other standard-of-care treatments (e.g., radiation therapy). We recommend that TPP+-based drugs that have been tested in preclinical models or in humans should undergo clinical trials in patients with cancer.
Conflict of interest statement
Declaration of Interest:
Balaraman Kalyanaraman is an inventor of US Patent No. 9,956,233, “Neuroprotection by mitochondria-targeted metformin.”
Balaraman Kalyanaraman and Micael Hardy are inventors of US Patent No. 11,274,114, “Modified mito-metformin compounds and methods of synthesis and use thereof”; US Patent No. 10,836,782, “Mito-honokiol compounds and methods of synthesis and use thereof”; and US Patent No. 11,352,382, “Mito-lonidamine, compositions and methods of use.”
Balaraman Kalyanaraman, Gang Cheng, and Micael Hardy are inventors of US Patent No. 11,083,739 and US Patent No. 11,612,610, both titled “Mito-magnolol compounds and methods of synthesis and use thereof,” and WO2021081500A1, “Mitochondria-targeted atovaquone: a more potent and more effective antitumor, antimicrobial, and antimalarial drug,” which is under consideration by the US Patent and Trademark Office.
Figures
Similar articles
-
Exploiting the tumor immune microenvironment and immunometabolism using mitochondria-targeted drugs: Challenges and opportunities in racial disparity and cancer outcome research.FASEB J. 2022 Apr;36(4):e22226. doi: 10.1096/fj.202101862R. FASEB J. 2022. PMID: 35233843 Free PMC article. Review.
-
Characterizing OXPHOS inhibitor-mediated alleviation of hypoxia using high-throughput live cell-imaging.Cancer Metab. 2024 May 3;12(1):13. doi: 10.1186/s40170-024-00342-6. Cancer Metab. 2024. PMID: 38702787 Free PMC article.
-
Combining PEGylated mito-atovaquone with MCT and Krebs cycle redox inhibitors as a potential strategy to abrogate tumor cell proliferation.Sci Rep. 2022 Mar 24;12(1):5143. doi: 10.1038/s41598-022-08984-6. Sci Rep. 2022. PMID: 35332210 Free PMC article.
-
Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives.Acta Pharm Sin B. 2018 Oct;8(6):862-880. doi: 10.1016/j.apsb.2018.05.006. Epub 2018 May 18. Acta Pharm Sin B. 2018. PMID: 30505656 Free PMC article. Review.
-
Mitochondria Targeting of Oxidative Phosphorylation Inhibitors to Alleviate Hypoxia and Enhance Anticancer Treatment Efficacy.Clin Cancer Res. 2025 Apr 1;31(7):1186-1193. doi: 10.1158/1078-0432.CCR-24-3296. Clin Cancer Res. 2025. PMID: 39898881 Review.
Cited by
-
The troglitazone derivative EP13 disrupts energy metabolism through respiratory chain complex I inhibition in breast cancer cells and potentiates the antiproliferative effect of glycolysis inhibitors.Cancer Cell Int. 2024 Apr 10;24(1):132. doi: 10.1186/s12935-024-03319-z. Cancer Cell Int. 2024. PMID: 38594745 Free PMC article.
-
Metabolic crossroads: unravelling immune cell dynamics in gastrointestinal cancer drug resistance.Cancer Drug Resist. 2025 Feb 8;8:7. doi: 10.20517/cdr.2024.164. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40051496 Free PMC article. Review.
-
A Mitochondria-Targeting SIRT3 Inhibitor with Activity against Diffuse Large B Cell Lymphoma.J Med Chem. 2024 Sep 12;67(17):15428-15437. doi: 10.1021/acs.jmedchem.4c01053. Epub 2024 Aug 27. J Med Chem. 2024. PMID: 39191393 Free PMC article.
-
Tumor energy metabolism: implications for therapeutic targets.Mol Biomed. 2024 Nov 29;5(1):63. doi: 10.1186/s43556-024-00229-4. Mol Biomed. 2024. PMID: 39609317 Free PMC article. Review.
-
Serine metabolism in tumor progression and immunotherapy.Discov Oncol. 2025 Apr 28;16(1):628. doi: 10.1007/s12672-025-02358-w. Discov Oncol. 2025. PMID: 40295433 Free PMC article. Review.
References
-
-
Yap TA, Daver N, Mahendra M, et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat Med. 2023. 2023/01/01;29(1):115–126.
* Neurotoxicity was reported in cancer patients treated with an OXPHOS inhibitor
-
-
-
Zhang X, Dang CV. Time to hit pause on mitochondria-targeting cancer therapies. Nat Med. 2023. 2023/01/01;29(1):29–30.
** Editorial on halting clinical trials using mitochondrial-targeted therapies
-
-
- Ashton TM, McKenna WG, Kunz-Schughart LA, et al. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res. 2018. Jun 1;24(11):2482–2490. - PubMed
-
-
Huang M, Myers CR, Wang Y, et al. Mitochondria as a Novel Target for Cancer Chemoprevention: Emergence of Mitochondrial-targeting Agents. Cancer Prev Res (Phila). 2021. Mar;14(3):285–306.
* Review article describing the use of mitochondria-targeted agents in chemoprevention
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
