The Potential microRNA Prognostic Signature in HNSCCs: A Systematic Review
- PMID: 37736900
- PMCID: PMC10514860
- DOI: 10.3390/ncrna9050054
The Potential microRNA Prognostic Signature in HNSCCs: A Systematic Review
Abstract
Head and neck squamous cell carcinomas (HNSCCs) are often diagnosed at advanced stages, incurring significant high mortality and morbidity. Several microRNAs (miRs) have been identified as pivotal players in the onset and advancement of HNSCCs, operating as either oncogenes or tumor suppressors. Distinctive miR patterns identified in tumor samples, as well as in serum, plasma, or saliva, from patients have significant clinical potential for use in the diagnosis and prognosis of HNSCCs and as potential therapeutic targets. The aim of this study was to identify previous systematic reviews with meta-analysis data and clinical trials that showed the most promising miRs in HNSCCs, enclosing them into a biomolecular signature to test the prognostic value on a cohort of HNSCC patients according to The Cancer Genome Atlas (TCGA). Three electronic databases (PubMed, Scopus, and Science Direct) and one registry (the Cochrane Library) were investigated, and a combination of keywords such as "signature microRNA OR miR" AND "HNSCC OR LSCC OR OSCC OR oral cancer" were searched. In total, 15 systematic literature reviews and 76 prognostic clinical reports were identified for the study design and inclusion process. All survival index data were extracted, and the three miRs (miR-21, miR-155, and miR-375) most investigated and presenting the largest number of patients included in the studies were selected in a molecular biosignature. The difference between high and low tissue expression levels of miR-21, miR-155, and miR-375 for OS had an HR = 1.28, with 95% CI: [0.95, 1.72]. In conclusion, the current evidence suggests that miRNAs have potential prognostic value to serve as screening tools for clinical practice in HNSCC follow-up and treatment. Further large-scale cohort studies focusing on these miRNAs are recommended to verify the clinical utility of these markers individually and/or in combination.
Keywords: HNSCC; microRNA; oral cancer; risk factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

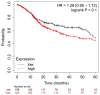
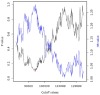
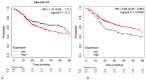
References
-
- Yashooa R.K., Nabi A.Q. The miR-146a-5p and miR-125b-5p levels as biomarkers for early prediction of Alzheimer’s disease. Hum. Gene. 2022;34:201129. doi: 10.1016/j.humgen.2022.201129. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

