Prenatal exposures to organophosphate ester metabolite mixtures and children's neurobehavioral outcomes in the MADRES pregnancy cohort
- PMID: 37737180
- PMCID: PMC10515433
- DOI: 10.1186/s12940-023-01017-3
Prenatal exposures to organophosphate ester metabolite mixtures and children's neurobehavioral outcomes in the MADRES pregnancy cohort
Abstract
Background: Evidence suggests organophosphate esters (OPEs) are neurotoxic; however, the epidemiological literature remains scarce. We investigated whether prenatal exposures to OPEs were associated with child neurobehavior in the MADRES cohort.
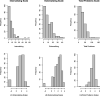
Methods: We measured nine OPE metabolites in 204 maternal urine samples (gestational age at collection: 31.4 ± 1.8 weeks). Neurobehavior problems were assessed among 36-month-old children using the Child Behavior Checklist's (CBCL) three composite scales [internalizing, externalizing, and total problems]. We examined associations between tertiles of prenatal OPE metabolites (> 50% detection) and detect/non-detect categories (< 50% detection) and CBCL composite scales using linear regression and generalized additive models. We also examined mixtures for widely detected OPEs (n = 5) using Bayesian kernel machine regression.
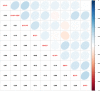
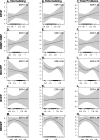
Results: Maternal participants with detectable versus non-detectable levels of bis(2-methylphenyl) phosphate (BMPP) had children with 42% (95% CI: 4%, 96%) higher externalizing, 45% (-2%, 114%) higher internalizing, and 35% (3%, 78%) higher total problems. Participants in the second versus first tertile of bis(butoxethyl) phosphate (BBOEP) had children with 43% (-1%, 109%) higher externalizing scores. Bis(1-chloro-2-propyl) phosphate (BCIPP) and child sex had a statistically significant interaction in internalizing (p = 0.02) and total problems (p = 0.03) models, with 120% (23%, 295%) and 57% (6%, 134%) higher scores in the third versus first BCIPP tertile among males. Among females, detectable vs non-detectable levels of prenatal BMPP were associated with 69% higher externalizing scores (5%, 170%) while the third versus first tertile of prenatal BBOEP was associated with 45% lower total problems (-68%, -6%). Although the metabolite mixture and each CBCL outcome had null associations, we observed marginal associations between di-n-butyl phosphate and di-isobutyl phosphate (DNBP + DIBP) and higher internalizing scores (0.15; 95% CrI: -0.02, 0.32), holding other metabolites at their median.
Conclusions: Our results generally suggest adverse and sex-specific effects of prenatal exposure to previously understudied OPEs on neurobehavioral outcomes in 36-month children, providing evidence of potential OPE neurotoxicity.
Keywords: Early childhood; Mixtures; Neurobehavior; OPE; OPFRs; Organophosphate esters.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Monk C, Hane A. Fetal and infant brain–behavior development: milestones & environmental influences. 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

