Circadian clock disruption promotes the degeneration of dopaminergic neurons in male Drosophila
- PMID: 37737209
- PMCID: PMC10516932
- DOI: 10.1038/s41467-023-41540-y
Circadian clock disruption promotes the degeneration of dopaminergic neurons in male Drosophila
Abstract
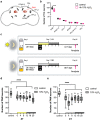
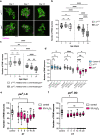
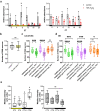
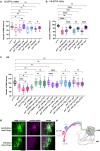
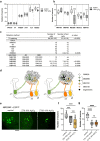
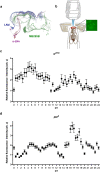
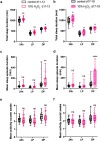
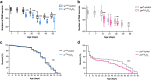
Sleep and circadian rhythm disruptions are frequent comorbidities of Parkinson's disease (PD), a disorder characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra. However, the causal role of circadian clocks in the degenerative process remains uncertain. We demonstrated here that circadian clocks regulate the rhythmicity and magnitude of the vulnerability of DA neurons to oxidative stress in male Drosophila. Circadian pacemaker neurons are presynaptic to a subset of DA neurons and rhythmically modulate their susceptibility to degeneration. The arrhythmic period (per) gene null mutation exacerbates the age-dependent loss of DA neurons and, in combination with brief oxidative stress, causes premature animal death. These findings suggest that circadian clock disruption promotes dopaminergic neurodegeneration.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function.PLoS Genet. 2017 Jan 10;13(1):e1006507. doi: 10.1371/journal.pgen.1006507. eCollection 2017 Jan. PLoS Genet. 2017. PMID: 28072817 Free PMC article.
-
UNC79 and UNC80, putative auxiliary subunits of the NARROW ABDOMEN ion channel, are indispensable for robust circadian locomotor rhythms in Drosophila.PLoS One. 2013 Nov 5;8(11):e78147. doi: 10.1371/journal.pone.0078147. eCollection 2013. PLoS One. 2013. PMID: 24223770 Free PMC article.
-
Dissection of central clock function in Drosophila through cell-specific CRISPR-mediated clock gene disruption.Elife. 2019 Oct 15;8:e48308. doi: 10.7554/eLife.48308. Elife. 2019. PMID: 31613218 Free PMC article.
-
Circadian Rhythm Abnormalities in Parkinson's Disease from Humans to Flies and Back.Int J Mol Sci. 2018 Dec 6;19(12):3911. doi: 10.3390/ijms19123911. Int J Mol Sci. 2018. PMID: 30563246 Free PMC article. Review.
-
Neurogenetics of Drosophila circadian clock: expect the unexpected.J Neurogenet. 2017 Dec;31(4):250-265. doi: 10.1080/01677063.2017.1370466. Epub 2017 Sep 4. J Neurogenet. 2017. PMID: 28868955 Review.
Cited by
-
In vivo Effects of Salicornia herbacea and Calystegia soldanella Extracts for Memory Improvement.J Microbiol Biotechnol. 2024 May 28;34(5):1092-1100. doi: 10.4014/jmb.2312.12045. Epub 2024 Mar 13. J Microbiol Biotechnol. 2024. PMID: 38563091 Free PMC article.
-
The molecular clock drives motivated locomotion and time-of-day-dependent firing patterns in mouse dopaminergic neurons.NPJ Biol Timing Sleep. 2025;2(1):28. doi: 10.1038/s44323-025-00044-2. Epub 2025 Jul 3. NPJ Biol Timing Sleep. 2025. PMID: 40620297 Free PMC article.
-
Nighttime caffeine intake increases motor impulsivity.iScience. 2025 Jul 24;28(8):113197. doi: 10.1016/j.isci.2025.113197. eCollection 2025 Aug 15. iScience. 2025. PMID: 40809001 Free PMC article.
-
Circadian Influences on Brain Lipid Metabolism and Neurodegenerative Diseases.Metabolites. 2024 Dec 22;14(12):723. doi: 10.3390/metabo14120723. Metabolites. 2024. PMID: 39728504 Free PMC article. Review.
-
Altered reactivity to threatening stimuli in Drosophila models of Parkinson's disease, revealed by a trial-based assay.Elife. 2025 Jul 29;13:RP90905. doi: 10.7554/eLife.90905. Elife. 2025. PMID: 40728873 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

