MicroRNA-1 protects the endothelium in acute lung injury
- PMID: 37737266
- PMCID: PMC10561733
- DOI: 10.1172/jci.insight.164816
MicroRNA-1 protects the endothelium in acute lung injury
Abstract
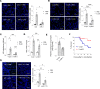
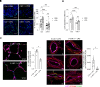
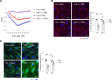
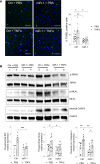
Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS), cause severe endothelial dysfunction in the lung, and vascular endothelial growth factor (VEGF) is elevated in ARDS. We found that the levels of a VEGF-regulated microRNA, microRNA-1 (miR-1), were reduced in the lung endothelium after acute injury. Pulmonary endothelial cell-specific (EC-specific) overexpression of miR-1 protected the lung against cell death and barrier dysfunction in both murine and human models and increased the survival of mice after pneumonia-induced ALI. miR-1 had an intrinsic protective effect in pulmonary and other types of ECs; it inhibited apoptosis and necroptosis pathways and decreased capillary leak by protecting adherens and tight junctions. Comparative gene expression analysis and RISC recruitment assays identified miR-1 targets in the context of injury, including phosphodiesterase 5A (PDE5A), angiopoietin-2 (ANGPT2), CNKSR family member 3 (CNKSR3), and TNF-α-induced protein 2 (TNFAIP2). We validated miR-1-mediated regulation of ANGPT2 in both mouse and human ECs and found that in a 119-patient pneumonia cohort, miR-1 correlated inversely with ANGPT2. These findings illustrate a previously unknown role of miR-1 as a cytoprotective orchestrator of endothelial responses to acute injury with prognostic and therapeutic potential.
Keywords: Endothelial cells; Noncoding RNAs; Pulmonology; Vascular Biology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

