A branching model of lineage differentiation underpinning the neurogenic potential of enteric glia
- PMID: 37737269
- PMCID: PMC10516949
- DOI: 10.1038/s41467-023-41492-3
A branching model of lineage differentiation underpinning the neurogenic potential of enteric glia
Abstract
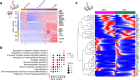
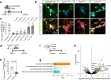
Glial cells have been proposed as a source of neural progenitors, but the mechanisms underpinning the neurogenic potential of adult glia are not known. Using single cell transcriptomic profiling, we show that enteric glial cells represent a cell state attained by autonomic neural crest cells as they transition along a linear differentiation trajectory that allows them to retain neurogenic potential while acquiring mature glial functions. Key neurogenic loci in early enteric nervous system progenitors remain in open chromatin configuration in mature enteric glia, thus facilitating neuronal differentiation under appropriate conditions. Molecular profiling and gene targeting of enteric glial cells in a cell culture model of enteric neurogenesis and a gut injury model demonstrate that neuronal differentiation of glia is driven by transcriptional programs employed in vivo by early progenitors. Our work provides mechanistic insight into the regulatory landscape underpinning the development of intestinal neural circuits and generates a platform for advancing glial cells as therapeutic agents for the treatment of neural deficits.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Furness, J. The Enteric Nervous System (Blackwell Publishing, 2006).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

