Tonsils are major sites of persistence of SARS-CoV-2 in children
- PMID: 37737615
- PMCID: PMC10581087
- DOI: 10.1128/spectrum.01347-23
Tonsils are major sites of persistence of SARS-CoV-2 in children
Abstract
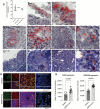
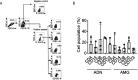
In the present study, we show that SARS-CoV-2 can infect palatine tonsils, adenoids, and secretions in children without symptoms of COVID-19, with no history of recent upper airway infection. We studied 48 children undergoing tonsillectomy due to snoring/OSA or recurrent tonsillitis between October 2020 and September 2021. Nasal cytobrushes, nasal washes, and tonsillar tissue fragments obtained at surgery were tested by RT-qPCR, immunohistochemistry (IHC), flow cytometry, and neutralization assay. We detected the presence of SARS-CoV-2 in at least one specimen tested in 27% of patients. IHC revealed the presence of the viral nucleoprotein in epithelial surface and in lymphoid cells in both extrafollicular and follicular regions, in adenoids and palatine tonsils. Also, IHC for the SARS-CoV-2 non-structural protein NSP-16 indicated the presence of viral replication in 53.8% of the SARS-CoV-2-infected tissues. Flow cytometry showed that CD20+ B lymphocytes were the most infected phenotypes, followed by CD4+ lymphocytes and CD123 dendritic cells, CD8+ T lymphocytes, and CD14+ macrophages. Additionally, IF indicated that infected tonsillar tissues had increased expression of ACE2 and TMPRSS2. NGS sequencing demonstrated the presence of different SARS-CoV-2 variants in tonsils from different tissues. SARS-CoV-2 antigen detection was not restricted to tonsils but was also detected in nasal cells from the olfactory region. Palatine tonsils and adenoids are sites of prolonged RNA presence by SARS-CoV-2 in children, even without COVID-19 symptoms. IMPORTANCE This study shows that SRS-CoV-2 of different lineages can infect tonsils and adenoids in one quarter of children undergoing tonsillectomy. These findings bring advancement to the area of SARS-CoV-2 pathogenesis, by showing that tonsils may be sites of prolonged infection, even without evidence of recent COVID-19 symptoms. SARS-CoV-2 infection of B and T lymphocytes, macrophages, and dendritic cells may interfere with the mounting of immune responses in these secondary lymphoid organs. Moreover, the shedding of SARS-CoV-2 RNA in respiratory secretions from silently infected children raises concern about possible diagnostic confusion in the presence of symptoms of acute respiratory infections caused by other etiologies.
Keywords: COVID-19; adenoid; children; otorhinolaryngology; palatine tonsil; pediatric infectious disease; persistent infection; respiratory viruses; viral infection; virus persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Silent Infection of B and CD8+ T Lymphocytes by Influenza A Virus in Children with Tonsillar Hypertrophy.J Virol. 2020 Apr 16;94(9):e01969-19. doi: 10.1128/JVI.01969-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075928 Free PMC article.
-
High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease.PLoS One. 2012;7(8):e42136. doi: 10.1371/journal.pone.0042136. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22870291 Free PMC article. Clinical Trial.
-
Impact of rapid palatal expansion on the size of adenoids and tonsils in children.Sleep Med. 2022 Apr;92:96-102. doi: 10.1016/j.sleep.2022.02.011. Epub 2022 Feb 19. Sleep Med. 2022. PMID: 35390750 Free PMC article.
-
Lymphoid changes of the nasopharyngeal and palatine tonsils that are indicative of human immunodeficiency virus infection. A clinicopathologic study of 12 cases.Am J Surg Pathol. 1996 May;20(5):572-87. doi: 10.1097/00000478-199605000-00004. Am J Surg Pathol. 1996. PMID: 8619422 Review.
-
The problems of tonsils and adenoids.J Singapore Paediatr Soc. 1989;31(3-4):97-102. J Singapore Paediatr Soc. 1989. PMID: 2700589 Review.
Cited by
-
Novel Findings on the Development and Immunological Functions of Palatine Tonsils.Clin Rev Allergy Immunol. 2025 Jun 18;68(1):58. doi: 10.1007/s12016-025-09071-0. Clin Rev Allergy Immunol. 2025. PMID: 40531272 Review.
-
Sex differences and immune correlates of Long COVID development, persistence, and resolution.bioRxiv [Preprint]. 2024 Jun 19:2024.06.18.599612. doi: 10.1101/2024.06.18.599612. bioRxiv. 2024. Update in: Sci Transl Med. 2024 Nov 13;16(773):eadr1032. doi: 10.1126/scitranslmed.adr1032. PMID: 38948732 Free PMC article. Updated. Preprint.
-
Antiviral and anti-inflammatory efficacy of nanoencapsulated brazilian green propolis against SARS-CoV-2.Sci Rep. 2025 Jul 1;15(1):21627. doi: 10.1038/s41598-025-05683-w. Sci Rep. 2025. PMID: 40596171 Free PMC article.
-
Vascular Pathogenesis in Acute and Long COVID: Current Insights and Therapeutic Outlook.Semin Thromb Hemost. 2025 Apr;51(3):256-271. doi: 10.1055/s-0044-1790603. Epub 2024 Sep 30. Semin Thromb Hemost. 2025. PMID: 39348850 Free PMC article. Review.
-
Mucosal Immunity against SARS-CoV-2 in the Respiratory Tract.Pathogens. 2024 Jan 26;13(2):113. doi: 10.3390/pathogens13020113. Pathogens. 2024. PMID: 38392851 Free PMC article. Review.
References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 588:E6. doi:10.1038/s41586-020-2951-z - DOI - PMC - PubMed
-
- Proenca-Modena JL, Pereira Valera FC, Jacob MG, Buzatto GP, Saturno TH, Lopes L, Souza JM, Escremim Paula F, Silva ML, Carenzi LR, Tamashiro E, Arruda E, Anselmo-Lima WT. 2012. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS One 7:e42136. doi:10.1371/journal.pone.0042136 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

