The Lysosomal Calcium Channel TRPML1 Maintains Mitochondrial Fitness in NK Cells through Interorganelle Cross-Talk
- PMID: 37737664
- PMCID: PMC10579149
- DOI: 10.4049/jimmunol.2300406
The Lysosomal Calcium Channel TRPML1 Maintains Mitochondrial Fitness in NK Cells through Interorganelle Cross-Talk
Abstract
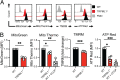
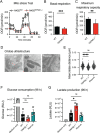
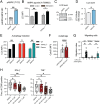
Cytotoxic lymphocytes eliminate cancer cells through the release of lytic granules, a specialized form of secretory lysosomes. This compartment is part of the pleomorphic endolysosomal system and is distinguished by its highly dynamic Ca2+ signaling machinery. Several transient receptor potential (TRP) calcium channels play essential roles in endolysosomal Ca2+ signaling and ensure the proper function of these organelles. In this study, we examined the role of TRPML1 (TRP cation channel, mucolipin subfamily, member 1) in regulating the homeostasis of secretory lysosomes and their cross-talk with mitochondria in human NK cells. We found that genetic deletion of TRPML1, which localizes to lysosomes in NK cells, led to mitochondrial fragmentation with evidence of collapsed mitochondrial cristae. Consequently, TRPML1-/- NK92 (NK92ML1-/-) displayed loss of mitochondrial membrane potential, increased reactive oxygen species stress, reduced ATP production, and compromised respiratory capacity. Using sensitive organelle-specific probes, we observed that mitochondria in NK92ML1-/- cells exhibited evidence of Ca2+ overload. Moreover, pharmacological activation of the TRPML1 channel in primary NK cells resulted in upregulation of LC3-II, whereas genetic deletion impeded autophagic flux and increased accumulation of dysfunctional mitochondria. Thus, TRPML1 impacts autophagy and clearance of damaged mitochondria. Taken together, these results suggest that an intimate interorganelle communication in NK cells is orchestrated by the lysosomal Ca2+ channel TRPML1.
Copyright © 2023 The Authors.
Conflict of interest statement
K.-J.M. is a consultant and has stock interests at Fate Therapeutics and Vycellix, is a member of the Scientific Advisory Board at Vycellix, and receives research support from Fate Therapeutics and Oncopeptides. The other authors have no financial conflicts of interest.
Figures


Similar articles
-
TRP Channels as Interior Designers: Remodeling the Endolysosomal Compartment in Natural Killer Cells.Front Immunol. 2020 Apr 28;11:753. doi: 10.3389/fimmu.2020.00753. eCollection 2020. Front Immunol. 2020. PMID: 32411146 Free PMC article. Review.
-
Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19266-19275. doi: 10.1073/pnas.2003236117. Epub 2020 Jul 23. Proc Natl Acad Sci U S A. 2020. PMID: 32703809 Free PMC article.
-
TRPML1-Emerging Roles in Cancer.Cells. 2020 Dec 13;9(12):2682. doi: 10.3390/cells9122682. Cells. 2020. PMID: 33322223 Free PMC article.
-
The lysosomal Ca2+ release channel TRPML1 regulates lysosome size by activating calmodulin.J Biol Chem. 2017 May 19;292(20):8424-8435. doi: 10.1074/jbc.M116.772160. Epub 2017 Mar 30. J Biol Chem. 2017. PMID: 28360104 Free PMC article.
-
Involvement of the TRPML Mucolipin Channels in Viral Infections and Anti-viral Innate Immune Responses.Front Immunol. 2020 Apr 29;11:739. doi: 10.3389/fimmu.2020.00739. eCollection 2020. Front Immunol. 2020. PMID: 32425938 Free PMC article. Review.
Cited by
-
The Golgi complex governs natural killer cell lytic granule positioning to promote directionality in cytotoxicity.Cell Rep. 2025 Jan 28;44(1):115156. doi: 10.1016/j.celrep.2024.115156. Epub 2025 Jan 14. Cell Rep. 2025. PMID: 39813120 Free PMC article.
-
Autophagy in tumor immune escape and immunotherapy.Mol Cancer. 2025 Mar 19;24(1):85. doi: 10.1186/s12943-025-02277-y. Mol Cancer. 2025. PMID: 40102867 Free PMC article. Review.
-
HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence.Proc Natl Acad Sci U S A. 2024 Jan 9;121(2):e2306454120. doi: 10.1073/pnas.2306454120. Epub 2024 Jan 3. Proc Natl Acad Sci U S A. 2024. PMID: 38170752 Free PMC article.
-
Lysosome-Mitochondrial Crosstalk in Cellular Stress and Disease.Antioxidants (Basel). 2025 Jan 22;14(2):125. doi: 10.3390/antiox14020125. Antioxidants (Basel). 2025. PMID: 40002312 Free PMC article. Review.
-
Interplay of α-Synuclein Oligomers and Endoplasmic Reticulum Stress in Parkinson'S Disease: Insights into Cellular Dysfunctions.Inflammation. 2025 Aug;48(4):1590-1606. doi: 10.1007/s10753-024-02156-6. Epub 2024 Oct 9. Inflammation. 2025. PMID: 39382817 Review.
References
-
- Gallo, E. M., Canté-Barrett K., Crabtree G. R.. 2006. Lymphocyte calcium signaling from membrane to nucleus. Nat. Immunol. 7: 25–32. - PubMed
-
- Berridge, M. J., Lipp P., Bootman M. D.. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1: 11–21. - PubMed
-
- Davis, L. C., Morgan A. J., Galione A.. 2022. Acidic Ca2+ stores and immune-cell function. Cell Calcium 101: 102516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

