Analysis of Pregnancy Outcomes Following Exposure to Intramuscular Interferon Beta-1a: The AVONEX® Pregnancy Exposure Registry
- PMID: 37737962
- PMCID: PMC10730480
- DOI: 10.1007/s40801-023-00384-0
Analysis of Pregnancy Outcomes Following Exposure to Intramuscular Interferon Beta-1a: The AVONEX® Pregnancy Exposure Registry
Abstract
Background and objectives: There is a lack of well-controlled US studies of intramuscular (IM) interferon beta (IFNβ)-1a use in pregnant women with multiple sclerosis; however, in the European Medicines Agency region, IFNβ formulations may be considered during pregnancy if clinically needed based on data from European Union cohort registries. The AVONEX Pregnancy Exposure Registry was established to prospectively study the effects of IM IFNβ-1a on the risk of birth defects and spontaneous pregnancy loss in a US population.
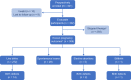
Methods: Pregnant women with multiple sclerosis exposed to IM IFNβ-1a within ~ 1 week of conception or during the first trimester were included. Participants were followed until there was a pregnancy outcome, live-born infants were followed until age 8-12 weeks. Data were collected on IM IFNβ-1a exposure, demographics, patient characteristics, medical history, and pregnancy outcomes, including live births (with or without birth defect), spontaneous abortions/miscarriages and fetal death/stillbirth, elective abortions (with and without birth defect), and ectopic pregnancies. A population-based birth defect surveillance program, the Metropolitan Atlanta Congenital Defects Program (MACDP), served as the primary external control group for evaluating the risk of birth defects.
Results: Three-hundred and two patients with a median (range) age of 31.0 (16-48) years and a median (range) gestational age at the time of enrollment of 10.1 (4-39) weeks were evaluable. Most patients (n = 278/302; 92%) reported IM IFNβ-1a exposure in the week before conception and most (n = 293/302; 97%) discontinued treatment before the end of the first trimester. Of 306 pregnancy outcomes, there were 272 live births, 28 spontaneous abortions of 266 pregnancies enrolled before 22 weeks' gestation (rate 10.5%; 95% confidence interval 7.2-15.0), five elective abortions, and one stillbirth. There were 17 adjudicator-confirmed major birth defects of 272 live births (rate 6.3%; 95% confidence interval 3.8-10.0); the pattern of birth defects observed was not suggestive of a relationship to prenatal IM IFNβ-1a exposure.
Conclusions: This large US registry study suggests IM IFNβ-1a exposure during early pregnancy was not clinically associated with adverse pregnancy outcomes in women with multiple sclerosis. These findings help inform clinicians and patients in weighing the risks and benefits of IM IFNβ-1a use during pregnancy.
Clinical trial registration: ClinicalTrials.gov: NCT00168714, 15 September, 2005.
© 2023. The Author(s).
Conflict of interest statement
Bianca Weinstock-Guttman has participated in speaker’s bureaus and/or served as a consultant for, and/or received grant/research support from Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Myers Squibb, Sanofi Genzyme, Bayer, Janssen, Labcorp, and Horizon. She serves on the editorial board of
Figures
References
-
- Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: prevalence and impact. Health Rep. 2018;29(1):3–8. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

