Laboratory-based X-ray spectrometer for actinide science
- PMID: 37738030
- PMCID: PMC10624025
- DOI: 10.1107/S1600577523006926
Laboratory-based X-ray spectrometer for actinide science
Abstract
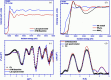
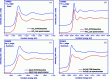
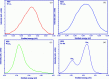
X-ray absorption and emission spectroscopies nowadays are advanced characterization methods for fundamental and applied actinide research. One of the advantages of these methods is to reveal slight changes in the structural and electronic properties of radionuclides. The experiments are generally carried out at synchrotrons. However, considerable progress has been made to construct laboratory-based X-ray spectrometers for X-ray absorption and emission spectroscopies. Laboratory spectrometers are reliable, effective and accessible alternatives to synchrotrons, especially for actinide research, which allow dispensing with high costs of the radioactive sample transport and synchrotron time. Moreover, data from laboratory spectrometers, obtained within a reasonable time, are comparable with synchrotron results. Thereby, laboratory spectrometers can complement synchrotrons or can be used for preliminary experiments to find perspective samples for synchrotron experiments with better resolution. Here, the construction and implementation of an X-ray spectrometer (LomonosovXAS) in Johann-geometry at a radiochemistry laboratory is reported. Examples are given of the application of LomonosovXAS to actinide systems relevant to the chemistry of f-elements, the physical chemistry of nuclear power engineering and the long-term disposal of spent nuclear fuel.
Keywords: X-ray absorption spectroscopy; X-ray emission spectroscopy; X-ray spectrometers; laboratory-based.
open access.
Figures



References
-
- Amidani, L., Plakhova, T. V., Romanchuk, A. Y., Gerber, E., Weiss, S., Efimenko, A., Sahle, C. J., Butorin, S. M., Kalmykov, S. N. & Kvashnina, K. O. (2019). Phys. Chem. Chem. Phys. 21, 10635–10643. - PubMed
-
- Bearden, J. A. & Burr, A. F. (1967). Rev. Mod. Phys. 39, 125–142.
-
- Bergmann, U. & Cramer, S. P. (1998). Proc. SPIE, 3448, https://doi.org/10.1117/12.332507.
-
- Bès, R., Ahopelto, T., Honkanen, A. P., Huotari, S., Leinders, G., Pakarinen, J. & Kvashnina, K. (2018). J. Nucl. Mater. 507, 50–53.
-
- Bes, R., Takala, S. & Huotari, S. (2021). Rev. Sci. Instrum. 92, 043106. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

