Low rate of nonrelapse mortality in under-4-year-olds with ALL given chemotherapeutic conditioning for HSCT: a phase 3 FORUM study
- PMID: 37738088
- PMCID: PMC10827403
- DOI: 10.1182/bloodadvances.2023010591
Low rate of nonrelapse mortality in under-4-year-olds with ALL given chemotherapeutic conditioning for HSCT: a phase 3 FORUM study
Abstract
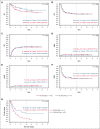
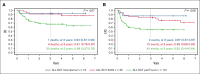
Allogeneic hematopoietic stem cell transplantation (HSCT) is highly effective for treating pediatric high-risk or relapsed acute lymphoblastic leukemia (ALL). For young children, total body irradiation (TBI) is associated with severe late sequelae. In the FORUM study (NCT01949129), we assessed safety, event-free survival (EFS), and overall survival (OS) of 2 TBI-free conditioning regimens in children aged <4 years with ALL. Patients received fludarabine (Flu), thiotepa (Thio), and either busulfan (Bu) or treosulfan (Treo) before HSCT. From 2013 to 2021, 191 children received transplantation and were observed for ≥6 months (median follow-up: 3 years). The 3-year OS was 0.63 (95% confidence interval [95% CI], 0.52-0.72) and 0.76 (95% CI, 0.64-0.84) for Flu/Thio/Bu and Flu/Thio/Treo (P = .075), respectively. Three-year EFS was 0.52 (95% CI, 0.41-0.61) and 0.51 (95% CI, 0.39-0.62), respectively (P = .794). Cumulative incidence of nonrelapse mortality (NRM) and relapse at 3 years were 0.06 (95% CI, 0.02-0.12) vs 0.03 (95% CI: <0.01-0.09) (P = .406) and 0.42 (95% CI, 0.31-0.52) vs 0.45 (95% CI, 0.34-0.56) (P = .920), respectively. Grade >1 acute graft-versus-host disease (GVHD) occurred in 29% of patients receiving Flu/Thio/Bu and 17% of those receiving Flu/Thio/Treo (P = .049), whereas grade 3/4 occurred in 10% and 9%, respectively (P = .813). The 3-year incidence of chronic GVHD was 0.07 (95% CI, 0.03-0.13) vs 0.05 (95% CI, 0.02-0.11), respectively (P = .518). In conclusion, both chemotherapeutic conditioning regimens were well tolerated and NRM was low. However, relapse was the major cause of treatment failure. This trial was registered at www.clinicaltrials.gov as #NCT01949129.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.B. declares research grants from Neovii, Riemser, Medac, and Bristol Myers Squibb (to the institution); is a member of advisory boards for Novartis, Celgene, Amgen, Medac, and Servier (personal and institutional); has received speaker fees from Miltenyi, Jazz, Riemser, Novartis, and Amgen (to the institution); and declares a patent with and royalties from Medac. M.A. has received speaker and traveling fees from Jazz. J.B. has participated in advisory boards for Amgen, Novartis, Pfizer, and Janssen (to the institution), and has received speaker fees from Novartis. T.G. has received research grants from Jazz. M.I. has received honoraria for an advisory board role from Novartis. H.P. declares travel grants from Neovii. T.T. has received honoraria for consultancy and advisory board roles in Servier and Jazz. F.L. is a member of advisory boards for Amgen, Novartis, Bellicum Pharmaceuticals, Neovii, and Vertex, and has received speaker fees from Amgen, Novartis, Miltenyi, Medac, Jazz Pharmaceuticals, and Takeda, outside the submitted work. C.P. declares research grants from Amgen, Neovii, Riemser, Medac, and Jazz; is a member of advisory boards for Amgen, Neovii, Jazz, and Novartis; and has received speaker fees from Amgen, Neovii, Novartis, Medac, and Riemser. The remaining authors declare no competing financial interests.
Figures
References
-
- von Stackelberg A, Volzke E, Kuhl JS, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer. 2011;47(1):90–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

