Gibberellin signaling regulates lignin biosynthesis to modulate rice seed shattering
- PMID: 37738159
- PMCID: PMC10689197
- DOI: 10.1093/plcell/koad244
Gibberellin signaling regulates lignin biosynthesis to modulate rice seed shattering
Abstract
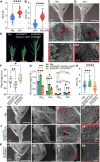
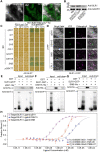
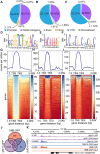
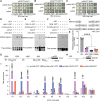
The elimination of seed shattering was a key step in rice (Oryza sativa) domestication. In this paper, we show that increasing the gibberellic acid (GA) content or response in the abscission region enhanced seed shattering in rice. We demonstrate that SLENDER RICE1 (SLR1), the key repressor of GA signaling, could physically interact with the rice seed shattering-related transcription factors quantitative trait locus of seed shattering on chromosome 1 (qSH1), O. sativa HOMEOBOX 15 (OSH15), and SUPERNUMERARY BRACT (SNB). Importantly, these physical interactions interfered with the direct binding of these three regulators to the lignin biosynthesis gene 4-COUMARATE: COENZYME A LIGASE 3 (4CL3), thereby derepressing its expression. Derepression of 4CL3 led to increased lignin deposition in the abscission region, causing reduced rice seed shattering. Importantly, we also show that modulating GA content could alter the degree of seed shattering to increase harvest efficiency. Our results reveal that the "Green Revolution" phytohormone GA is important for regulating rice seed shattering, and we provide an applicable breeding strategy for high-efficiency rice harvesting.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. The authors declare no competing interests.
Figures




Similar articles
-
KNOX Protein OSH15 Induces Grain Shattering by Repressing Lignin Biosynthesis Genes.Plant Physiol. 2017 May;174(1):312-325. doi: 10.1104/pp.17.00298. Epub 2017 Mar 28. Plant Physiol. 2017. PMID: 28351912 Free PMC article.
-
The APETALA2-Like Transcription Factor SUPERNUMERARY BRACT Controls Rice Seed Shattering and Seed Size.Plant Cell. 2019 Jan;31(1):17-36. doi: 10.1105/tpc.18.00304. Epub 2019 Jan 9. Plant Cell. 2019. PMID: 30626621 Free PMC article.
-
The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis.Plant J. 2014 Sep;79(5):717-28. doi: 10.1111/tpj.12581. Epub 2014 Jul 9. Plant J. 2014. PMID: 24923192
-
The BELL-KNOX module qSH1-OSH71 regulates rice seed shattering via direct activation of OsXTH12 xyloglucanase.Plant Physiol. 2025 Aug 4;198(4):kiaf320. doi: 10.1093/plphys/kiaf320. Plant Physiol. 2025. PMID: 40730354
-
Advances in Rice Seed Shattering.Int J Mol Sci. 2023 May 17;24(10):8889. doi: 10.3390/ijms24108889. Int J Mol Sci. 2023. PMID: 37240235 Free PMC article. Review.
Cited by
-
Identification of genetic loci for seed shattering in Italian ryegrass (Lolium multiflorum Lam.).Theor Appl Genet. 2024 Dec 24;138(1):11. doi: 10.1007/s00122-024-04801-y. Theor Appl Genet. 2024. PMID: 39718645
-
OsKANADI1 and OsYABBY5 regulate rice plant height by targeting GIBERELLIN 2-OXIDASE6.Plant Cell. 2024 Dec 23;37(1):koae276. doi: 10.1093/plcell/koae276. Plant Cell. 2024. PMID: 39383255
-
SHATTERING ABORTION3 controls rice seed shattering by promoting abscission zone separation.Plant Commun. 2025 May 12;6(5):101282. doi: 10.1016/j.xplc.2025.101282. Epub 2025 Feb 11. Plant Commun. 2025. PMID: 39943691 Free PMC article. No abstract available.
-
Genetic dissection of a reduced seed-shattering trait acquired in rice domestication.Breed Sci. 2024 Sep;74(4):285-294. doi: 10.1270/jsbbs.23080. Epub 2024 Aug 30. Breed Sci. 2024. PMID: 39872329 Free PMC article.
-
Abscission in plants: from mechanism to applications.Adv Biotechnol (Singap). 2024 Aug 9;2(3):27. doi: 10.1007/s44307-024-00033-9. Adv Biotechnol (Singap). 2024. PMID: 39883313 Free PMC article. Review.
References
-
- Bertolotti G, Unterholzner SJ, Scintu D, Salvi E, Svolacchia N, Di Mambro R, Ruta V, Linhares Scaglia F, Vittorioso P, Sabatini S, et al. . A PHABULOSA-controlled genetic pathway regulates ground tissue patterning in the Arabidopsis root. Curr Biol. 2021:31(2):420–426.e6. 10.1016/j.cub.2020.10.038 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 31901527/National Natural Science Foundation of China
- 2018M641555/China Postdoctoral Science Foundation
- JCYJ20170303154319837/Science Technology and Innovation Commission of Shenzhen Municipality
- 2020B1515120086/Guangdong Basic and Applied Basic Research Foundation
- 2020YFE0202300/National Key R&D Program
LinkOut - more resources
Full Text Sources
Research Materials

