Binding of herpesvirus entry mediator (HVEM) and HSV-1 gD affect reactivation but not latency levels
- PMID: 37738264
- PMCID: PMC10550154
- DOI: 10.1371/journal.ppat.1011693
Binding of herpesvirus entry mediator (HVEM) and HSV-1 gD affect reactivation but not latency levels
Abstract
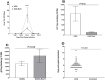
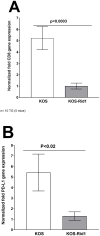
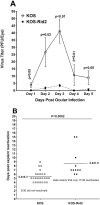
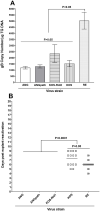
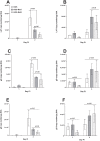
Previously we reported that the HSV-1 latency associated transcript (LAT) specifically upregulates the cellular herpesvirus entry mediator (HVEM) but no other known HSV-1 receptors. HSV-1 glycoprotein D (gD) binds to HVEM but the effect of this interaction on latency-reactivation is not known. We found that the levels of latent viral genomes were not affected by the absence of gD binding to HVEM. However, reactivation of latent virus in trigeminal ganglia explant cultures was blocked in the absence of gD binding to HVEM. Neither differential HSV-1 replication and spread in the eye nor levels of latency influenced reactivation. Despite similar levels of latency, reactivation in the absence of gD binding to HVEM correlated with reduced T cell exhaustion. Our results indicate that HVEM-gD signaling plays a significant role in HSV-1 reactivation but not in ocular virus replication or levels of latency. The results presented here identify gD binding to HVEM as an important target that influences reactivation and survival of ganglion resident T cells but not levels of latency. This concept may also apply to other herpesviruses that engages HVEM.
Copyright: © 2023 Jaggi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

