Brain-machine interface learning is facilitated by specific patterning of distributed cortical feedback
- PMID: 37738340
- PMCID: PMC10516504
- DOI: 10.1126/sciadv.adh1328
Brain-machine interface learning is facilitated by specific patterning of distributed cortical feedback
Abstract
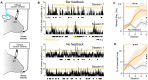
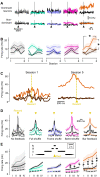
Neuroprosthetics offer great hope for motor-impaired patients. One obstacle is that fine motor control requires near-instantaneous, rich somatosensory feedback. Such distributed feedback may be recreated in a brain-machine interface using distributed artificial stimulation across the cortical surface. Here, we hypothesized that neuronal stimulation must be contiguous in its spatiotemporal dynamics to be efficiently integrated by sensorimotor circuits. Using a closed-loop brain-machine interface, we trained head-fixed mice to control a virtual cursor by modulating the activity of motor cortex neurons. We provided artificial feedback in real time with distributed optogenetic stimulation patterns in the primary somatosensory cortex. Mice developed a specific motor strategy and succeeded to learn the task only when the optogenetic feedback pattern was spatially and temporally contiguous while it moved across the topography of the somatosensory cortex. These results reveal spatiotemporal properties of the sensorimotor cortical integration that set constraints on the design of neuroprosthetics.
Figures





Similar articles
-
Learning in a closed-loop brain-machine interface with distributed optogenetic cortical feedback.J Neural Eng. 2022 Dec 29;19(6). doi: 10.1088/1741-2552/acab87. J Neural Eng. 2022. PMID: 36579369
-
Rapid Integration of Artificial Sensory Feedback during Operant Conditioning of Motor Cortex Neurons.Neuron. 2017 Feb 22;93(4):929-939.e6. doi: 10.1016/j.neuron.2017.01.023. Neuron. 2017. PMID: 28231470 Free PMC article.
-
A fast intracortical brain-machine interface with patterned optogenetic feedback.J Neural Eng. 2018 Aug;15(4):046011. doi: 10.1088/1741-2552/aabb80. Epub 2018 Apr 4. J Neural Eng. 2018. PMID: 29616982
-
Brain-machine interfaces for rehabilitation of poststroke hemiplegia.Prog Brain Res. 2016;228:163-83. doi: 10.1016/bs.pbr.2016.04.020. Epub 2016 Jun 29. Prog Brain Res. 2016. PMID: 27590969 Review.
-
Neural Plasticity in Sensorimotor Brain-Machine Interfaces.Annu Rev Biomed Eng. 2023 Jun 8;25:51-76. doi: 10.1146/annurev-bioeng-110220-110833. Epub 2023 Feb 28. Annu Rev Biomed Eng. 2023. PMID: 36854262 Free PMC article. Review.
References
-
- Johansson R. S., Westling G., Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp. Brain Res. 56, 550–564 (1984). - PubMed
-
- Monzée J., Lamarre Y., Smith A. M., The effects of digital anesthesia on force control using a precision grip. J. Neurophysiol. 89, 672–683 (2003). - PubMed
-
- Brochier T., Boudreau M.-J., Paré M., Smith A. M., The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp. Brain Res. 128, 31–40 (1999). - PubMed
-
- Chesler A. T., Szczot M., Bharucha-Goebel D., Čeko M., Donkervoort S., Laubacher C., Hayes L. H., Alter K., Zampieri C., Stanley C., Innes A. M., Mah J. K., Grosmann C. M., Bradley N., Nguyen D., Foley A. R., Le Pichon C. E., Bönnemann C. G., The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355–1364 (2016). - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

