Galectin-3 Cooperates with CD47 to Suppress Phagocytosis and T-cell Immunity in Gastric Cancer Peritoneal Metastases
- PMID: 37738407
- PMCID: PMC10843008
- DOI: 10.1158/0008-5472.CAN-23-0783
Galectin-3 Cooperates with CD47 to Suppress Phagocytosis and T-cell Immunity in Gastric Cancer Peritoneal Metastases
Abstract
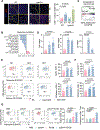
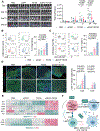
The peritoneal cavity is a common site of gastric adenocarcinoma (GAC) metastasis. Peritoneal carcinomatosis (PC) is resistant to current therapies and confers poor prognosis, highlighting the need to identify new therapeutic targets. CD47 conveys a "don't eat me" signal to myeloid cells upon binding its receptor signal regulatory protein alpha (SIRPα), which helps tumor cells circumvent macrophage phagocytosis and evade innate immune responses. Previous studies demonstrated that the blockade of CD47 alone results in limited clinical benefits, suggesting that other target(s) might need to be inhibited simultaneously with CD47 to elicit a strong antitumor response. Here, we found that CD47 was highly expressed on malignant PC cells, and elevated CD47 was associated with poor prognosis. Galectin-3 (Gal3) expression correlated with CD47 expression, and coexpression of Gal3 and CD47 was significantly associated with diffuse type, poor differentiation, and tumor relapse. Depletion of Gal3 reduced expression of CD47 through inhibition of c-Myc binding to the CD47 promoter. Furthermore, injection of Gal3-deficient tumor cells into either wild-type and Lgals3-/- mice led to a reduction in M2 macrophages and increased T-cell responses compared with Gal3 wild-type tumor cells, indicating that tumor cell-derived Gal3 plays a more important role in GAC progression and phagocytosis than host-derived Gal3. Dual blockade of Gal3 and CD47 collaboratively suppressed tumor growth, increased phagocytosis, repolarized macrophages, and boosted T-cell immune responses. These data uncovered that Gal3 functions together with CD47 to suppress phagocytosis and orchestrate immunosuppression in GAC with PC, which supports exploring a novel combination therapy targeting Gal3 and CD47.
Significance: Dual inhibition of CD47 and Gal3 enhances tumor cell phagocytosis and reprograms macrophages to overcome the immunosuppressive microenvironment and suppress tumor growth in peritoneal metastasis of gastric adenocarcinoma.
©2023 American Association for Cancer Research.
Conflict of interest statement
Conflict interests:
The authors confirm that there are no conflicts of interest to disclose.
Figures




References
-
- Tanaka Y, Chiwaki F, Kojima S, Kawazu M, Komatsu M, Ueno T, et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer 2021;2:962–77 - PubMed
-
- Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018;17:887–904 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

