Adipocyte Glucocorticoid Receptor Inhibits Immune Regulatory Genes to Maintain Immune Cell Homeostasis in Adipose Tissue
- PMID: 37738419
- PMCID: PMC10558062
- DOI: 10.1210/endocr/bqad143
Adipocyte Glucocorticoid Receptor Inhibits Immune Regulatory Genes to Maintain Immune Cell Homeostasis in Adipose Tissue
Abstract
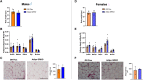
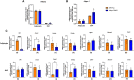
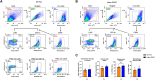
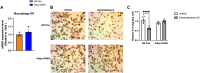
Glucocorticoids acting via the glucocorticoid receptors (GR) are key regulators of metabolism and the stress response. However, uncontrolled or excessive GR signaling adversely affects adipose tissue, including endocrine, immune, and metabolic functions. Inflammation of the adipose tissue promotes systemic metabolic dysfunction; however, the molecular mechanisms underlying the role of adipocyte GR in regulating genes associated with adipose tissue inflammation are poorly understood. We performed in vivo studies using adipocyte-specific GR knockout mice in conjunction with in vitro studies to understand the contribution of adipocyte GR in regulating adipose tissue immune homeostasis. Our findings show that adipocyte-specific GR signaling regulates adipokines at both mRNA and plasma levels and immune regulatory (Coch, Pdcd1, Cemip, and Cxcr2) mRNA gene expression, which affects myeloid immune cell presence in white adipose tissue. We found that, in adipocytes, GR directly influences Cxcr2. This chemokine receptor promotes immune cell migration, indirectly affecting Pdcd1 and Cemip gene expression in nonadipocyte or stromal cells. Our findings suggest that GR adipocyte signaling suppresses inflammatory signals, maintaining immune homeostasis. We also found that GR signaling in adipose tissue in response to stress is sexually dimorphic. Understanding the molecular relationship between GR signaling and adipose tissue inflammation could help develop potential targets to improve local and systemic inflammation, insulin sensitivity, and metabolic health.
Keywords: adipose tissue; adipose tissue homeostasis; glucocorticoid receptor; glucocorticoids.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth.J Endocrinol. 2016 Jul;230(1):1-11. doi: 10.1530/JOE-16-0121. Epub 2016 Apr 22. J Endocrinol. 2016. PMID: 27106108
-
Loss of epidermal glucocorticoid receptor protects against whole body metabolic dysfunction upon chronic corticosterone treatment.Mol Metab. 2023 Aug;74:101763. doi: 10.1016/j.molmet.2023.101763. Epub 2023 Jun 24. Mol Metab. 2023. PMID: 37364709 Free PMC article.
-
The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses.J Endocrinol. 2010 Feb;204(2):153-64. doi: 10.1677/JOE-09-0292. Epub 2009 Nov 25. J Endocrinol. 2010. PMID: 19939912
-
Effects of glucocorticoids on adipose tissue plasticity.Ann Endocrinol (Paris). 2024 Jun;85(3):259-262. doi: 10.1016/j.ando.2024.05.013. Epub 2024 Jun 12. Ann Endocrinol (Paris). 2024. PMID: 38871499 Review.
-
Endogenous Glucocorticoid Signaling in the Regulation of Bone and Marrow Adiposity: Lessons from Metabolism and Cross Talk in Other Tissues.Curr Osteoporos Rep. 2019 Dec;17(6):438-445. doi: 10.1007/s11914-019-00554-6. Curr Osteoporos Rep. 2019. PMID: 31749087 Review.
Cited by
-
Deficiency of glucocorticoid receptor in bone marrow adipocytes has mild effects on bone and hematopoiesis but does not influence expansion of marrow adiposity with caloric restriction.Front Endocrinol (Lausanne). 2024 Jun 3;15:1397081. doi: 10.3389/fendo.2024.1397081. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38887268 Free PMC article.
References
-
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243‐251. - PubMed
-
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions*. Endocr Rev. 2000;21(1):55‐89. - PubMed
-
- Swarbrick M, Zhou H, Seibel M. Mechanisms in endocrinology: local and systemic effects of glucocorticoids on metabolism: new lessons from animal models. Eur J Endocrinol. 2021;185(5):R113‐R129. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

