The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer's Disease
- PMID: 37738628
- PMCID: PMC10692438
- DOI: 10.1093/gerona/glad226
The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer's Disease
Abstract
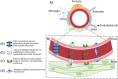
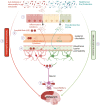
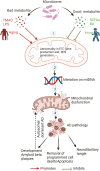
Alzheimer's disease (AD) is a progressive, age-related neurodegenerative disorder that affects a large proportion of the older population. It currently lacks effective treatments, placing a heavy burden on patients, families, health care systems, and society. This is mainly due to our limited comprehension of the pathophysiology of AD progression, as well as the lack of effective drug targets and intervention timing to address the underlying pathology. AD is a multifactorial condition, and emerging evidence suggests that abnormalities in the gut microbiota play a significant role as environmental and multifaceted contributors to AD, although the exact mechanisms are yet to be fully explored. Changes in the composition of microbiota influence host neuronal health through their metabolites. These metabolites regulate intestinal epithelia, blood-brain barrier permeability, and neuroinflammation by affecting mitochondrial function. The decline in the proportion of beneficial microbes and their essential metabolites during aging and AD is directly linked to poor mitochondrial function, although the specific mechanisms remain unclear. In this review, we discuss recent developments in understanding the impact of the microbiome and its metabolites on various cell types, their influence on the integrity of the gut and blood-brain barriers, systemic and brain inflammation, and cell-specific effects in AD pathology. This information is expected to pave the way for a new understanding of the interactions between microbiota and mitochondria in AD, providing a foundation for the development of novel treatments for AD.
Keywords: Alzheimer’s disease; Metabolites; Microbiome; Mitochondria.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Dr. Yadav is Co-Founder and Chief Scientific Officer of the Postbiotics Inc, but his role has not conflict with work presented in this manuscript. Other authors have no conflict of interest to report.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

