High-throughput screening of the Saccharomyces cerevisiae genome for 2-amino-3-methylimidazo [4,5-f] quinoline resistance identifies colon cancer-associated genes
- PMID: 37738679
- PMCID: PMC11025384
- DOI: 10.1093/g3journal/jkad219
High-throughput screening of the Saccharomyces cerevisiae genome for 2-amino-3-methylimidazo [4,5-f] quinoline resistance identifies colon cancer-associated genes
Abstract
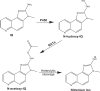
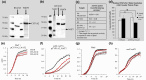
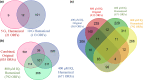
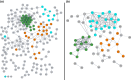
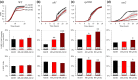
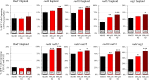
Heterocyclic aromatic amines (HAAs) are potent carcinogenic agents found in charred meats and cigarette smoke. However, few eukaryotic resistance genes have been identified. We used Saccharomyces cerevisiae (budding yeast) to identify genes that confer resistance to 2-amino-3-methylimidazo[4,5-f] quinoline (IQ). CYP1A2 and NAT2 activate IQ to become a mutagenic nitrenium compound. Deletion libraries expressing human CYP1A2 and NAT2 or no human genes were exposed to either 400 or 800 µM IQ for 5 or 10 generations. DNA barcodes were sequenced using the Illumina HiSeq 2500 platform and statistical significance was determined for exactly matched barcodes. We identified 424 ORFs, including 337 genes of known function, in duplicate screens of the "humanized" collection for IQ resistance; resistance was further validated for a select group of 51 genes by growth curves, competitive growth, or trypan blue assays. Screens of the library not expressing human genes identified 143 ORFs conferring resistance to IQ per se. Ribosomal protein and protein modification genes were identified as IQ resistance genes in both the original and "humanized" libraries, while nitrogen metabolism, DNA repair, and growth control genes were also prominent in the "humanized" library. Protein complexes identified included the casein kinase 2 (CK2) and histone chaperone (HIR) complex. Among DNA Repair and checkpoint genes, we identified those that function in postreplication repair (RAD18, UBC13, REV7), base excision repair (NTG1), and checkpoint signaling (CHK1, PSY2). These studies underscore the role of ribosomal protein genes in conferring IQ resistance, and illuminate DNA repair pathways for conferring resistance to activated IQ.
Keywords: budding yeast; colon cancer; genome profiling; heterocyclic aromatic amine.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflict of interest.
Figures
Similar articles
-
Effect of rapid human N-acetyltransferase 2 haplotype on DNA damage and mutagenesis induced by 2-amino-3-methylimidazo-[4,5-f]quinoline (IQ) and 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline (MeIQx).Mutat Res. 2010 Feb 3;684(1-2):66-73. doi: 10.1016/j.mrfmmm.2009.12.001. Epub 2009 Dec 11. Mutat Res. 2010. PMID: 20004212 Free PMC article.
-
Development and characterization of CHO repair-proficient cell lines for comparative mutagenicity and metabolism of heterocyclic amines from cooked food.Environ Mol Mutagen. 2003;41(1):7-13. doi: 10.1002/em.10125. Environ Mol Mutagen. 2003. PMID: 12552587
-
Prenylflavonoids from hops inhibit the metabolic activation of the carcinogenic heterocyclic amine 2-amino-3-methylimidazo[4, 5-f]quinoline, mediated by cDNA-expressed human CYP1A2.Drug Metab Dispos. 2000 Nov;28(11):1297-302. Drug Metab Dispos. 2000. PMID: 11038156
-
Metabolism of heterocyclic aromatic amines and strategies of human biomonitoring.Princess Takamatsu Symp. 1995;23:59-68. Princess Takamatsu Symp. 1995. PMID: 8844796 Review.
-
Biological significance of trace levels of mutagenic heterocyclic aromatic amines in human diet: a critical review.Food Chem Toxicol. 1994 Oct;32(10):977-94. doi: 10.1016/0278-6915(94)90093-0. Food Chem Toxicol. 1994. PMID: 7959450 Review.
References
-
- Ausubel FM. 2003. Current Protocols in Molecular Biology. New York: Greene Pub. Associates. p. 1.7.1–1.7.15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
