A multi-country analysis of COVID-19 hospitalizations by vaccination status
- PMID: 37738979
- PMCID: PMC10935543
- DOI: 10.1016/j.medj.2023.08.005
A multi-country analysis of COVID-19 hospitalizations by vaccination status
Abstract
Background: Individuals vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), when infected, can still develop disease that requires hospitalization. It remains unclear whether these patients differ from hospitalized unvaccinated patients with regard to presentation, coexisting comorbidities, and outcomes.
Methods: Here, we use data from an international consortium to study this question and assess whether differences between these groups are context specific. Data from 83,163 hospitalized COVID-19 patients (34,843 vaccinated, 48,320 unvaccinated) from 38 countries were analyzed.
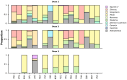
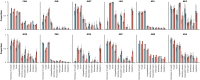
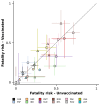
Findings: While typical symptoms were more often reported in unvaccinated patients, comorbidities, including some associated with worse prognosis in previous studies, were more common in vaccinated patients. Considerable between-country variation in both in-hospital fatality risk and vaccinated-versus-unvaccinated difference in this outcome was observed.
Conclusions: These findings will inform allocation of healthcare resources in future surges as well as design of longer-term international studies to characterize changes in clinical profile of hospitalized COVID-19 patients related to vaccination history.
Funding: This work was made possible by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z, 222410/Z/21/Z, 225288/Z/22/Z, and 220757/Z/20/Z); the Bill & Melinda Gates Foundation (OPP1209135); and the philanthropic support of the donors to the University of Oxford's COVID-19 Research Response Fund (0009109). Additional funders are listed in the "acknowledgments" section.
Keywords: COVID-19; Translation to population health; comorbidity; descriptive epidemiology; heterogeneity; vaccination.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests I.M.-L. declares lectures for Gilead, Thermo Fisher, and MSD; advisory board participation for Fresenius Kabi, Advanz Pharma, Gilead, Accelerate, and Merck; and consulting fees for Gilead outside of the submitted work. S.S. participated as an investigator for an observational study analyzing ICU patients with COVID-19 (for the Critical Care Consortium, including ECMOCARD) funded by The Prince Charles Hospital Foundation during the conduct of this study. B.L. declares travel/accommodation/meeting expenses from Mylan and Gilead, all outside the submitted work.
Figures



References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

