Xpert MTB/RIF Ultra on contaminated liquid cultures for tuberculosis and rifampicin-resistance detection: a diagnostic accuracy evaluation
- PMID: 37739001
- PMCID: PMC10600950
- DOI: 10.1016/S2666-5247(23)00169-6
Xpert MTB/RIF Ultra on contaminated liquid cultures for tuberculosis and rifampicin-resistance detection: a diagnostic accuracy evaluation
Abstract
Background: Xpert MTB/RIF Ultra (Ultra) is a widely used rapid front-line tuberculosis and rifampicin-susceptibility testing. Mycobacterium Growth Indicator Tube (MGIT) 960 liquid culture is used as an adjunct but is vulnerable to contamination. We aimed to assess whether Ultra can be used on to-be-discarded contaminated cultures.
Methods: We stored contaminated MGIT960 tubes (growth-positive, acid-fast bacilli [AFB]-negative) originally inoculated at a high-volume laboratory in Cape Town, South Africa, to diagnose patients with presumptive pulmonary tuberculosis. Patients who had no positive tuberculosis results (smear, Ultra, or culture) at contamination detection and had another, later specimen submitted within 3 months of the contaminated specimen were selected. We evaluated the sensitivity and specificity of Ultra on contaminated growth from the first culture for tuberculosis (next-available non-contaminated culture result reference standard) and rifampicin resistance (vs MTBDRplus on a later isolate). We calculated potential time-to-diagnosis improvements and also evaluated the immunochromatographic MPT64 TBc assay.
Findings: Between June 1 and Aug 31, 2019, 36 684 specimens from 26 929 patients were processed for diagnostic culture. 2402 (7%) cultures from 2186 patients were contaminated. 1068 (49%) of 2186 patients had no other specimen submitted. After 319 exclusions, there were 799 people with at least one repeat specimen submitted; of these, we included in our study 246 patients (31%) with a culture-positive repeat specimen and 429 patients (54%) with a culture-negative repeat specimen. 124 patients (16%) with a culture-contaminated repeat specimen were excluded. When Ultra was done on the initial contaminated growth, sensitivity was 89% (95% CI 84-94) for tuberculosis and 95% (75-100) for rifampicin-resistance detection, and specificity was 95% (90-98) for tuberculosis and 98% (93-100) for rifampicin-resistance detection. If our approach were used the day after contamination detection, the time to tuberculosis detection would improve by a median of 23 days (IQR 13-45) and provide a result in many patients who had none. MPT64 TBc had a sensitivity of 5% (95% CI 0-25).
Interpretation: Ultra on AFB-negative growth from contaminated MGIT960 tubes had high sensitivity and specificity, approximating WHO criteria for sputum test target product performance and exceeding drug susceptibility testing. Our approach could mitigate negative effects of culture contamination, especially when repeat specimens are not submitted.
Funding: The European & Developing Countries Clinical Trials Partnership, National Institutes of Health.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests GT reports funding from the EDCTP2 programme supported by the EU (RIA2018D-2509, PreFIT; RIA2018D-2493, SeroSelectTB; RIA2020I-3305, CAGE-TB) and the National Institutes of Health (D43TW010350; U01AI152087; U54EB027049; R01AI136894). RW reports funding from the South African Medical Research Council. All other authors report no competing interests.
Figures

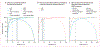
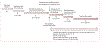
References
-
- WHO. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. 2017. https://apps.who.int/iris/bitstream/handle/10665/254792/WHO-HTM-TB-20?se... (accessed Feb 28, 2023).
-
- WHO. WHO operational handbook on tuberculosis, module 3 diagnosis, rapid diagnostics for tuberculosis detection. 2021. https://apps.who.int/iris/handle/10665/342369 (accessed Feb 28, 2023).
-
- Department of Health, Republic of South Africa. Symptom-based integrated approach to the adult in primary care. 2020. https://www.knowledgehub.org.za/system/files/elibdownloads/2020-08/APC%2... (accessed Feb 28, 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

