Dysregulation of the progranulin-driven autophagy-lysosomal pathway mediates secretion of the nuclear protein TDP-43
- PMID: 37739033
- PMCID: PMC10641265
- DOI: 10.1016/j.jbc.2023.105272
Dysregulation of the progranulin-driven autophagy-lysosomal pathway mediates secretion of the nuclear protein TDP-43
Abstract
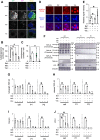
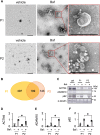
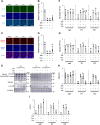
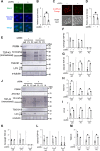
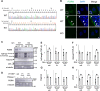
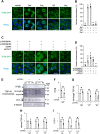
The cytoplasmic accumulation of the nuclear protein transactive response DNA-binding protein 43 kDa (TDP-43) has been linked to the progression of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. TDP-43 secreted into the extracellular space has been suggested to contribute to the cell-to-cell spread of the cytoplasmic accumulation of TDP-43 throughout the brain; however, the underlying mechanisms remain unknown. We herein demonstrated that the secretion of TDP-43 was stimulated by the inhibition of the autophagy-lysosomal pathway driven by progranulin (PGRN), a causal protein of frontotemporal lobar degeneration. Among modulators of autophagy, only vacuolar-ATPase inhibitors, such as bafilomycin A1 (Baf), increased the levels of the full-length and cleaved forms of TDP-43 and the autophagosome marker LC3-II (microtubule-associated proteins 1A/1B light chain 3B) in extracellular vesicle fractions prepared from the culture media of HeLa, SH-SY5Y, or NSC-34 cells, whereas vacuolin-1, MG132, chloroquine, rapamycin, and serum starvation did not. The C-terminal fragment of TDP-43 was required for Baf-induced TDP-43 secretion. The Baf treatment induced the translocation of the aggregate-prone GFP-tagged C-terminal fragment of TDP-43 and mCherry-tagged LC3 to the plasma membrane. The Baf-induced secretion of TDP-43 was attenuated in autophagy-deficient ATG16L1 knockout HeLa cells. The knockdown of PGRN induced the secretion of cleaved TDP-43 in an autophagy-dependent manner in HeLa cells. The KO of PGRN in mouse embryonic fibroblasts increased the secretion of the cleaved forms of TDP-43 and LC3-II. The treatment inducing TDP-43 secretion increased the nuclear translocation of GFP-tagged transcription factor EB, a master regulator of the autophagy-lysosomal pathway in SH-SY5Y cells. These results suggest that the secretion of TDP-43 is promoted by dysregulation of the PGRN-driven autophagy-lysosomal pathway.
Keywords: TAR DNA-binding protein 43; amyotrophic lateral sclerosis; autolysosome; autophagosome; autophagy; extracellular vesicles; frontotemporal lobar degeneration; lysosome; progranulin.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. - PubMed
-
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- Ratti A., Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016;138:95–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

