Enterobacteriaceae Growth Promotion by Intestinal Acylcarnitines, a Biomarker of Dysbiosis in Inflammatory Bowel Disease
- PMID: 37739064
- PMCID: PMC10694575
- DOI: 10.1016/j.jcmgh.2023.09.005
Enterobacteriaceae Growth Promotion by Intestinal Acylcarnitines, a Biomarker of Dysbiosis in Inflammatory Bowel Disease
Abstract
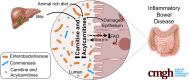
Background & aims: Altered plasma acylcarnitine levels are well-known biomarkers for a variety of mitochondrial fatty acid oxidation disorders and can be used as an alternative energy source for the intestinal epithelium when short-chain fatty acids are low. These membrane-permeable fatty acid intermediates are excreted into the gut lumen via bile and are increased in the feces of patients with inflammatory bowel disease (IBD).
Methods: Herein, based on studies in human subjects, animal models, and bacterial cultures, we show a strong positive correlation between fecal carnitine and acylcarnitines and the abundance of Enterobacteriaceae in IBD where they can be consumed by bacteria both in vitro and in vivo.
Results: Carnitine metabolism promotes the growth of Escherichia coli via anaerobic respiration dependent on the cai operon, and acetylcarnitine dietary supplementation increases fecal carnitine levels with enhanced intestinal colonization of the enteric pathogen Citrobacter rodentium.
Conclusions: In total, these results indicate that the increased luminal concentrations of carnitine and acylcarnitines in patients with IBD may promote the expansion of pathobionts belonging to the Enterobacteriaceae family, thereby contributing to disease pathogenesis.
Keywords: Carnitine; IBD; Metabolism; Microbiota.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Koves T.R., Ussher J.R., Noland R.C., et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

