The role of ferroptosis in cell-to-cell propagation of cell death initiated from focal injury in cardiomyocytes
- PMID: 37739163
- PMCID: PMC10591893
- DOI: 10.1016/j.lfs.2023.122113
The role of ferroptosis in cell-to-cell propagation of cell death initiated from focal injury in cardiomyocytes
Abstract
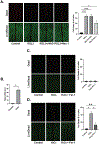
Aims: Ferroptosis has grown in importance as a key factor in ischemia-reperfusion (I/R) injury. This study explores the mechanism underlying fibrotic scarring extending along myofibers in cardiac ischemic injury and demonstrates the integral role of ferroptosis in causing a unique cell death pattern linked to I/R injury.
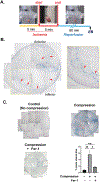
Main methods: Cadaveric hearts from individuals who had ischemic injury were examined by histological assays. We created a novel model of inducing cell death in H9c2 cells, and used it to demonstrate ferroptotic cell death extending in a cell-to-cell manner. Ex vivo Langendorff-perfused hearts were used alongside the model to replicate cell death extension along myofibers while also demonstrating protective effects of a ferroptosis inhibitor, ferrostatin-1 (Fer-1).
Key findings: Human hearts from individuals who had I/R injury demonstrated scarring along myofibers that was consistent with mouse models, suggesting that cell death extended from cell-to-cell. Treatment with Ras-selective lethal 3 (RSL3), a ferroptosis inducer, and exposure to excess iron exacerbated cell death propagation in in vitro models, and inhibition of ferroptosis by Fer-1 blunted this effect in both settings. In ex vivo models, Fer-1 was sufficient to reduce cell death along the myofibers caused by external injury.
Significance: The unique I/R injury-induced pattern of cell death along myofibers requires novel injury models that mimic this phenomenon, thus we established new methods to replicate it. Ferroptosis is important in propagating injury between cells and better understanding this mechanism may lead to therapeutic responses that limit I/R injury.
Keywords: Cardiomyocytes; Cell death; Ferroptosis; Myocardial infarction; Reperfusion injury.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “The role of ferroptosis in cell-to-cell propagation of cell death initiated from focal injury in cardiomyocytes”.
Figures





Similar articles
-
Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes.Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H659-H668. doi: 10.1152/ajpheart.00452.2017. Epub 2017 Nov 10. Am J Physiol Heart Circ Physiol. 2018. PMID: 29127238 Free PMC article.
-
Gossypol Acetic Acid Attenuates Cardiac Ischemia/Reperfusion Injury in Rats via an Antiferroptotic Mechanism.Biomolecules. 2021 Nov 10;11(11):1667. doi: 10.3390/biom11111667. Biomolecules. 2021. PMID: 34827665 Free PMC article.
-
Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury.Am J Physiol Heart Circ Physiol. 2021 Mar 1;320(3):H1170-H1184. doi: 10.1152/ajpheart.00237.2020. Epub 2021 Jan 29. Am J Physiol Heart Circ Physiol. 2021. PMID: 33513080
-
Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury.Int J Mol Sci. 2024 Jan 11;25(2):897. doi: 10.3390/ijms25020897. Int J Mol Sci. 2024. PMID: 38255971 Free PMC article. Review.
-
Broadening horizons: The role of ferroptosis in myocardial ischemia-reperfusion injury.Naunyn Schmiedebergs Arch Pharmacol. 2023 Oct;396(10):2269-2286. doi: 10.1007/s00210-023-02506-5. Epub 2023 Apr 29. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37119287 Review.
Cited by
-
A Closed Circulation Langendorff Heart Perfusion Method for Cardiac Drug Screening.Physiol Res. 2024 Dec 31;73(6):951-961. doi: 10.33549/physiolres.935324. Physiol Res. 2024. PMID: 39903886 Free PMC article.
-
Galectin-13 reduces membrane localization of SLC7A11 for ferroptosis propagation.Nat Chem Biol. 2025 Apr 17. doi: 10.1038/s41589-025-01888-2. Online ahead of print. Nat Chem Biol. 2025. PMID: 40246981
-
Iron Overload-induced Ferroptosis as a Target for Protection against Obliterative Bronchiolitis after Orthotopic Tracheal Transplantation in Mice.Curr Mol Med. 2025;25(6):746-759. doi: 10.2174/0115665240304363240524103203. Curr Mol Med. 2025. PMID: 38835130 Free PMC article.
-
Cell Death, by Any Other Name….Cells. 2024 Feb 10;13(4):325. doi: 10.3390/cells13040325. Cells. 2024. PMID: 38391938 Free PMC article. Review.
-
Butylated Hydroxytoluene (BHT) Protects SH-SY5Y Neuroblastoma Cells from Ferroptotic Cell Death: Insights from In Vitro and In Vivo Studies.Antioxidants (Basel). 2024 Feb 17;13(2):242. doi: 10.3390/antiox13020242. Antioxidants (Basel). 2024. PMID: 38397840 Free PMC article.
References
-
- Katz MY, Kusakari Y, Aoyagi H, Higa JK, Xiao CY, Abdelkarim AZ, Marh K, Aoyagi T, Rosenzweig A, Lozanoff S, Matsui T, Three-dimensional myocardial scarring along myofibers after coronary ischemia-reperfusion revealed by computerized images of histological assays, Physiol Rep 2(7) (2014). 10.14814/phy2.12072 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

