Two-year clinical outcomes of drug-coated balloon angioplasty and angiographic predictors of restenosis among patients with de novo femoropopliteal lesions
- PMID: 37739220
- PMCID: PMC10774568
- DOI: 10.1016/j.ihj.2023.09.002
Two-year clinical outcomes of drug-coated balloon angioplasty and angiographic predictors of restenosis among patients with de novo femoropopliteal lesions
Abstract
Objectives: We analyzed the 2-year clinical outcomes of patients with de novo femoropopliteal (FP) lesions who underwent drug-coated balloon (DCB) angioplasty and the angiographic predictors of restenosis.
Methods: This single-center, retrospective, and observational study evaluated 129 de novo FP lesions treated with DCB angioplasty without bailout stenting. Clinical outcomes and risk factors for loss of primary patency were analyzed using univariate and multivariate Cox proportional hazards regression models.
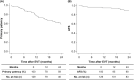
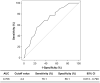
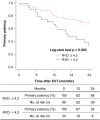
Results: The participants were aged 48-93 (mean: 73.6 ± 9.8) years, and 31% were women. Approximately 33% of the patients were receiving regular dialysis, and 35% of the affected limbs had critical ischemia. The mean lesion length was 132 ± 96 mm, and the mean reference vessel diameter (RVD) was 4.7 ± 0.8 mm. Forty-three (33%) limbs had chronic total occlusion of the target artery segment. Fifty-seven (44%) and 72 (56%) lesions were treated with DCB angioplasty using IN.PACT Admiral and Lutonix, respectively. The primary patency and amputation-free survival at 2 years were 59.3% and 89.5%, respectively. RVD was found to be an independent predictor of loss of primary patency. Based on the receiver operating characteristic analysis, an RVD of 4.2 mm was the best predictor of loss of primary patency at 2 years.
Conclusions: The short-term clinical outcome of DCB angioplasty for de novo FP lesions was acceptable. Moreover, an RVD of <4.2 mm was an independent predictor of restenosis after DCB angioplasty.
Keywords: Drug-coated balloon angioplasty; Endovascular treatment; Femoropopliteal artery.
Copyright © 2023. Published by Elsevier, a division of RELX India, Pvt. Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Aboyans V., Ricco J.B., Bartelink M.L., et al. ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur Heart J. 2017;39:763–816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

