Vancomycin-Induced Liver Injury, DRESS, and HLA-A∗32:01
- PMID: 37739311
- PMCID: PMC10885131
- DOI: 10.1016/j.jaip.2023.09.011
Vancomycin-Induced Liver Injury, DRESS, and HLA-A∗32:01
Abstract
Background: Intravenous vancomycin therapy can cause liver injury as well as "drug reaction with eosinophilia and systemic symptoms" (DRESS) syndrome. This study aimed to better define the clinical features and HLA associations of vancomycin-induced liver injury.
Objective: To describe clinical, biochemical, and temporal characteristics of vancomycin-induced liver injury.
Methods: Cases of liver injury with recent exposure to vancomycin who were enrolled in the US Drug-induced Liver Injury Network between 2004 and 2020 were assessed. Sequencing of HLA alleles was performed on stored blood samples.
Results: Among 1697 cases of drug-induced liver injury identified between 2004 and 2021, 9 (0.5%) were attributed to intravenous vancomycin. The 9 cases included 6 men, median age 60 years (range, 23-85 days), and treatment for 26 days (range, 1-34 days). The clinical presentation was DRESS syndrome in 8 patients, of whom 6 received corticosteroids. Liver injury varied from hepatocellular to cholestatic and from mild (n = 5) to fatal (n = 1). In survivors, liver injury and DRESS syndrome ultimately resolved. HLA typing demonstrated the HLA-A∗32:01 allele in 7 vancomycin cases (78%, all with DRESS syndrome), versus 1 of 81 cases (1.2%) exposed but not attributed to vancomycin, and 113 of 1708 cases (6.6%) without vancomycin exposure. The allele frequency in vancomycin cases was 0.44 compared with less than 0.04 in US populations.
Conclusions: Vancomycin-induced liver injury is commonly associated with DRESS syndrome and linked to HLA-A∗32:01. HLA-A∗32:01 testing could be considered early to risk-stratify patients using long-term intravenous vancomycin therapy.
Keywords: DRESS syndrome; Drug-induced liver injury; HLA testing; HLA-A∗32:01; Vancomycin.
Published by Elsevier Inc.
Conflict of interest statement
Figures
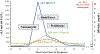
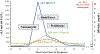
References
-
- Geraci JE, Nichols DR, Wellman WE. Vancomycin in serious Staphylococcal infections. Arch Intern Med 1962; 109: 507–515. - PubMed
-
- Levine DP. Vancomycin: a history. Clin Infect Dis 2006;42(Suppl 1):S5–S12. - PubMed
-
- Wilhelm MP, Estes L. Vancomycin. Mayo Clin Proc 1999; 74: 928–935. - PubMed
-
- Cadle RM, Mansouri MD. Vancomycin-induced elevation of liver enzyme levels. Ann Pharmacother 2006; 40: 1186–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

