Themis controls T cell activation, effector functions, and metabolism of peripheral CD8+ T cells
- PMID: 37739454
- PMCID: PMC10517225
- DOI: 10.26508/lsa.202302156
Themis controls T cell activation, effector functions, and metabolism of peripheral CD8+ T cells
Abstract
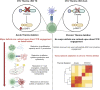
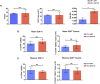
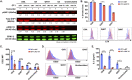
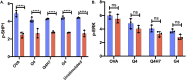
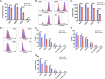
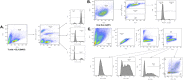
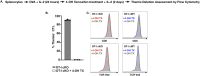
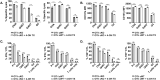
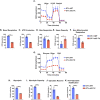
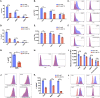
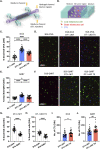
Themis is important in regulating positive selection of thymocytes during T cell development, but its role in peripheral T cells is less understood. Here, we investigated T cell activation and its sequelae using a tamoxifen-mediated, acute Themis deletion mouse model. We find that proliferation, effector functions including anti-tumor killing, and up-regulation of energy metabolism are severely compromised. This study reveals the phenomenon of peripheral adaptation to loss of Themis, by demonstrating direct TCR-induced defects after acute deletion of Themis that were not evident in peripheral T cells chronically deprived of Themis in dLck-Cre deletion model. Peripheral adaptation to long-term loss was compared using chronic versus acute tamoxifen-mediated deletion and with the (chronic) dLck-Cre deletion model. We found that upon chronic tamoxifen-mediated Themis deletion, there was modulation in the gene expression profile for both TCR and cytokine signaling pathways. This profile overlapped with (chronic) dLck-Cre deletion model. Hence, we found that peripheral adaptation induced changes to both TCR and cytokine signaling modules. Our data highlight the importance of Themis in the activation of CD8+ T cells.
© 2023 Gautam et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Themis is indispensable for IL-2 and IL-15 signaling in T cells.Sci Signal. 2022 Feb 15;15(721):eabi9983. doi: 10.1126/scisignal.abi9983. Epub 2022 Feb 15. Sci Signal. 2022. PMID: 35167340
-
Thymic-specific regulation of TCR signaling by Tespa1.Cell Mol Immunol. 2019 Dec;16(12):897-907. doi: 10.1038/s41423-019-0259-4. Epub 2019 Jul 17. Cell Mol Immunol. 2019. PMID: 31316154 Free PMC article.
-
THEMIS increases TCR signaling in CD4+CD8+ thymocytes by inhibiting the activity of the tyrosine phosphatase SHP1.Sci Signal. 2023 May 9;16(784):eade1274. doi: 10.1126/scisignal.ade1274. Epub 2023 May 9. Sci Signal. 2023. PMID: 37159521 Free PMC article.
-
Negative times negative equals positive, THEMIS sets the rule on thymic selection and peripheral T cell responses.Biomed J. 2022 Apr;45(2):334-346. doi: 10.1016/j.bj.2022.03.008. Epub 2022 Mar 26. Biomed J. 2022. PMID: 35346866 Free PMC article. Review.
-
THEMIS: a critical TCR signal regulator for ligand discrimination.Curr Opin Immunol. 2015 Apr;33:86-92. doi: 10.1016/j.coi.2015.01.020. Epub 2015 Feb 18. Curr Opin Immunol. 2015. PMID: 25700024 Review.
Cited by
-
Themis differentially regulates T follicular helper cell differentiation during early and late stages of chronic viral infection.Front Immunol. 2025 Jul 24;16:1638178. doi: 10.3389/fimmu.2025.1638178. eCollection 2025. Front Immunol. 2025. PMID: 40777021 Free PMC article.
-
The Ups and Downs of Metabolism during the Lifespan of a T Cell.Int J Mol Sci. 2020 Oct 27;21(21):7972. doi: 10.3390/ijms21217972. Int J Mol Sci. 2020. PMID: 33120978 Free PMC article. Review.
-
THEMIS promotes T cell development and maintenance by rising the signaling threshold of the inhibitory receptor BTLA.Proc Natl Acad Sci U S A. 2024 May 14;121(20):e2318773121. doi: 10.1073/pnas.2318773121. Epub 2024 May 7. Proc Natl Acad Sci U S A. 2024. PMID: 38713628 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
