Preventing Long-Term Brain Damage by Nerve Agent-Induced Status Epilepticus in Rat Models Applicable to Infants: Significant Neuroprotection by Tezampanel Combined with Caramiphen but Not by Midazolam Treatment
- PMID: 37739807
- PMCID: PMC10801760
- DOI: 10.1124/jpet.123.001710
Preventing Long-Term Brain Damage by Nerve Agent-Induced Status Epilepticus in Rat Models Applicable to Infants: Significant Neuroprotection by Tezampanel Combined with Caramiphen but Not by Midazolam Treatment
Abstract
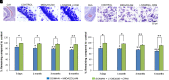
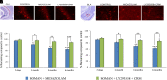
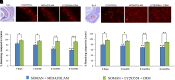
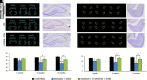
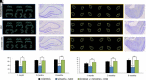
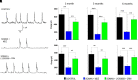
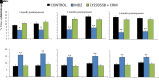
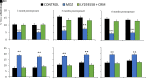
Acute exposure to nerve agents induces a peripheral cholinergic crisis and prolonged status epilepticus (SE), causing death or long-term brain damage. To provide preclinical data pertinent to the protection of infants and newborns, we compared the antiseizure and neuroprotective effects of treating soman-induced SE with midazolam (MDZ) versus tezampanel (LY293558) in combination with caramiphen (CRM) in 12- and 7-day-old rats. The anticonvulsants were administered 1 hour after soman exposure; neuropathology data were collected up to 6 months postexposure. In both ages, the total duration of SE within 24 hours after soman exposure was significantly shorter in the LY293558 plus CRM groups compared with the MDZ groups. Neuronal degeneration was substantial in the MDZ-treated groups but absent or minimal in the groups treated with LY293558 plus CRM. Loss of neurons and interneurons in the basolateral amygdala and CA1 hippocampal area was significant in the MDZ-treated groups but virtually absent in the LY293558 plus CRM groups. Atrophy of the amygdala and hippocampus occurred only in MDZ-treated groups. Neuronal/interneuronal loss and atrophy of the amygdala and hippocampus deteriorated over time. Reduction of inhibitory activity in the basolateral amygdala and increased anxiety were found only in MDZ groups. Spontaneous recurrent seizures developed in the MDZ groups, deteriorating over time; a small percentage of rats from the LY293558 plus CRM groups also developed seizures. These results suggest that brain damage can be long lasting or permanent if nerve agent-induced SE in infant victims is treated with midazolam at a delayed timepoint after SE onset, whereas antiglutamatergic treatment with tezampanel and caramiphen provides significant neuroprotection. SIGNIFICANCE STATEMENT: To protect the brain and the lives of infants in a mass exposure to nerve agents, an anticonvulsant treatment must be administered that will effectively stop seizures and prevent neuropathology, even if offered with a relative delay after seizure onset. The present study shows that midazolam, which was recently approved by the Food and Drug Administration for the treatment of nerve agent-induced status epilepticus, is not an effective neuroprotectant, whereas brain damage can be prevented by targeting glutamate receptors.
U.S. Government work not protected by U.S. copyright.
Figures


Similar articles
-
Delayed tezampanel and caramiphen treatment but not midazolam protects against long-term neuropathology after soman exposure.Exp Biol Med (Maywood). 2023 Apr;248(7):612-623. doi: 10.1177/15353702231171911. Epub 2023 Jun 10. Exp Biol Med (Maywood). 2023. PMID: 37300407 Free PMC article.
-
Electroencephalographic analysis in soman-exposed 21-day-old rats and the effects of midazolam or LY293558 with caramiphen.Ann N Y Acad Sci. 2020 Nov;1479(1):122-133. doi: 10.1111/nyas.14331. Epub 2020 Mar 31. Ann N Y Acad Sci. 2020. PMID: 32237259
-
Full Protection Against Soman-Induced Seizures and Brain Damage by LY293558 and Caramiphen Combination Treatment in Adult Rats.Neurotox Res. 2018 Oct;34(3):511-524. doi: 10.1007/s12640-018-9907-1. Epub 2018 Apr 30. Neurotox Res. 2018. PMID: 29713995
-
Targeting the glutamatergic system to counteract organophosphate poisoning: A novel therapeutic strategy.Neurobiol Dis. 2020 Jan;133:104406. doi: 10.1016/j.nbd.2019.02.017. Epub 2019 Feb 21. Neurobiol Dis. 2020. PMID: 30798006 Review.
-
Rational polytherapy in the treatment of cholinergic seizures.Neurobiol Dis. 2020 Jan;133:104537. doi: 10.1016/j.nbd.2019.104537. Epub 2019 Aug 24. Neurobiol Dis. 2020. PMID: 31454548 Review.
Cited by
-
Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition.Toxics. 2023 Oct 18;11(10):866. doi: 10.3390/toxics11100866. Toxics. 2023. PMID: 37888716 Free PMC article. Review.
-
Progress and Challenges in Developing Medical Countermeasures for Chemical, Biological, Radiological, and Nuclear Threat Agents.J Pharmacol Exp Ther. 2024 Jan 17;388(2):260-267. doi: 10.1124/jpet.123.002040. J Pharmacol Exp Ther. 2024. PMID: 38233227 Free PMC article.
References
-
- Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. - PubMed
-
- Andreollo NA, Santos EF, Araújo MR, Lopes LR (2012) Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig 25:49–51. - PubMed
-
- Aroniadou-Anderjaska V, Apland JP, Figueiredo TH, De Araujo Furtado M, Braga MF (2020) Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology 181:108298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
