Natalizumab Treatment Induces Proinflammatory CD4 T Cells Preferentially in the Integrin β7+ Compartment
- PMID: 37739811
- PMCID: PMC10519437
- DOI: 10.1212/NXI.0000000000200166
Natalizumab Treatment Induces Proinflammatory CD4 T Cells Preferentially in the Integrin β7+ Compartment
Erratum in
-
Corrections to Preprint Server Information.Neurol Neuroimmunol Neuroinflamm. 2024 Jul;11(4):e200267. doi: 10.1212/NXI.0000000000200267. Epub 2024 May 16. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38754048 Free PMC article. No abstract available.
Abstract
Background and objectives: Natalizumab, a monoclonal humanized antibody targeting integrin α4, inhibits the transmigration of lymphocytes into the CNS by preventing the interaction of integrin α4β1 with V-CAM expressed on brain vascular endothelial cells. Although natalizumab treatment reduces the clinical relapse rate in patients with relapsing-remitting MS, its discontinuation after reactivation of the JC virus is associated with a rebound of the disease in 20% of patients. The mechanisms of this rebound are not elucidated, but natalizumab increases the frequencies of circulating CD4 T cells expressing proinflammatory cytokines as well as the proportion of circulating Th17/Th1 cells (Th1-like Th17 cells). Gut-derived memory CD4 T cells are a population of growing interest in the pathogenesis of MS, but whether and how their properties are affected by natalizumab is not known. Here, we studied the phenotype and cytokine expression profile of circulating gut-derived memory CD4 T cells in patients with relapsing-remitting MS under natalizumab.
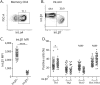
Methods: We identified gut-derived memory CD4 T cells by their expression of integrin β7 and compared their properties and those of integrin β7- memory CD4 T cells across healthy donors and patients with relapsing-remitting MS treated or not with natalizumab. We also compared the capacity of integrin β7- and integrin β7+ CD4 T-cell subsets to transmigrate in vitro across a model of blood-brain barrier.
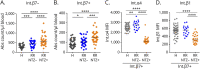
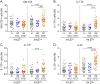
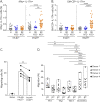
Results: The proportions of proinflammatory Th17/Th1 cells as well as of IL-17A+IFNγ+ and IL-17A+GM-CSF+ cells were higher in memory CD4 T cells expressing integrin β7 in patients receiving natalizumab compared with healthy donors and patients with relapsing-remitting MS not receiving natalizumab. By contrast, integrin β7 negative memory CD4 T cells only presented a modest increased in their proportion of Th17/Th1 cells under natalizumab. We further observed that integrin β7+ Th17/Th1 cells migrated as efficiently as integrin β7- Th17/Th1 across a monolayer of brain microvascular endothelial cells.
Discussion: Our study shows that circulating integrin β7+ memory CD4 T cells of patients with relapsing-remitting MS under natalizumab are enriched in proinflammatory cells supporting the hypothesis that integrin β7+ memory CD4 T cells could play a pathogenic role in the disease rebound observed at natalizumab discontinuation.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
B. Brochet reports grants, research support, personal fees or nonfinancial support from Genzyme, Bayer, Medday, Actelion, Roche, Biogen, Celgene, Novartis, and Merck outside the submitted work; A. Ruet or her institution reports grants, research support, personal fees or nonfinancial support from Genzyme, Bayer, Roche, Biogen, Novartis, and Merck outside the submitted work. Go to
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
