PRecision Event Monitoring for PatienTs with Heart Failure using HeartLogic (PREEMPT-HF) study design and enrolment
- PMID: 37740424
- PMCID: PMC10682906
- DOI: 10.1002/ehf2.14469
PRecision Event Monitoring for PatienTs with Heart Failure using HeartLogic (PREEMPT-HF) study design and enrolment
Abstract
Aims: The HeartLogic multisensor index has been found to be a sensitive predictor of worsening heart failure (HF). However, there is limited data on this index's association and its constituent sensors with HF readmissions.
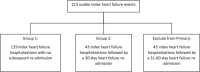
Methods and results: The PREEMPT-HF study is a global, multicentre, prospective, observational, single-arm, post-market study. HF patients with an implantable defibrillator device or cardiac resynchronization therapy with defibrillator with HeartLogic capabilities were eligible if sensor data collection was turned on and the HeartLogic feature was not enabled. Thus, the HeartLogic Index/alert and heart sounds sensor trends were unavailable via the LATITUDE remote monitoring system to clinicians (blinded). Evaluation of subject medical records at 6 months and a final in-clinic visit at 12 months was required for collection of all-cause hospitalizations and HF outpatient visits. The purpose of this study is exploratory, no formal hypothesis tests are planned, and no adjustment for multiple testing will be performed. A total of 2183 patients were enrolled at 103 sites between June 2018 and June 2020. A significant proportion of the patients were implanted with implantable defibrillator devices (39%) versus cardiac resynchronization therapy with defibrillator (61%); were female (27%); over 65 (61%); New York Heart Association class I (13%), II (53%), and III (33%); ejection fraction < 25% (21%); ischaemic (50%); and with a history of renal dysfunction (23%).
Conclusions: The PREEMPT study will provide clinical data and blinded sensor trends for the characterization of sensor changes with HF readmission, tachyarrhythmias, and event subgroups. These data may help to refine the clinical use of HeartLogic and to improve patient outcomes.
Trial registration: ClinicalTrials.gov 03579641.
Keywords: Diagnostic; Heart failure; Management; Prognosis; Readmissions.
© 2023 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
John Boehmer has received research funding from Abbott industries and compensation for consulting from Boston Scientific, Medtronic, Nanowear, and Zoll Medical Corporation. Roy Gardner and Andrew J. Sauer have received research funding and compensation for speaking and advising for Boston Scientific. Craig Stolen, Brian Kwan, Ramesh Wariar, and Stephen Ruble are Employees of Boston Scientific.
Figures
References
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol. 2014; 63: 1123–1133. - PubMed
-
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009; 53: 557–573. - PubMed
-
- Gravely‐Witte S, Jurgens CY, Tamim H, Grace SL. Length of delay in seeking medical care by patients with heart failure symptoms and the role of symptom‐related factors: a narrative review. Eur J Heart Fail. 2010; 12: 1122–1129. - PubMed
-
- Gardner RS, Thakur P, Hammill EF, Nair DG, Eldadah Z, Stančák B, Ferrick K, Sriratanasathavorn C, Duray GZ, Wariar R, Zhang Y, An Q, Averina V, Boehmer JP. Multiparameter diagnostic sensor measurements during clinically stable periods and worsening heart failure in ambulatory patients. ESC Heart Fail. 2021; 8: 1571–1581. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous