TSH and FT4 Reference Interval Recommendations and Prevalence of Gestational Thyroid Dysfunction: Quantification of Current Diagnostic Approaches
- PMID: 37740543
- PMCID: PMC10876390
- DOI: 10.1210/clinem/dgad564
TSH and FT4 Reference Interval Recommendations and Prevalence of Gestational Thyroid Dysfunction: Quantification of Current Diagnostic Approaches
Abstract
Context: Guidelines recommend use of population- and trimester-specific thyroid-stimulating hormone (TSH) and free thyroxine (FT4) reference intervals (RIs) in pregnancy. Since these are often unavailable, clinicians frequently rely on alternative diagnostic strategies. We sought to quantify the diagnostic consequences of current recommendations.
Methods: We included cohorts participating in the Consortium on Thyroid and Pregnancy. Different approaches were used to define RIs: a TSH fixed upper limit of 4.0 mU/L (fixed limit approach), a fixed subtraction from the upper limit for TSH of 0.5 mU/L (subtraction approach) and using nonpregnancy RIs. Outcome measures were sensitivity and false discovery rate (FDR) of women for whom levothyroxine treatment was indicated and those for whom treatment would be considered according to international guidelines.
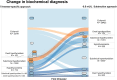
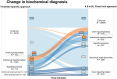
Results: The study population comprised 52 496 participants from 18 cohorts. Compared with the use of trimester-specific RIs, alternative approaches had a low sensitivity (0.63-0.82) and high FDR (0.11-0.35) to detect women with a treatment indication or consideration. Sensitivity and FDR to detect a treatment indication in the first trimester were similar between the fixed limit, subtraction, and nonpregnancy approach (0.77-0.11 vs 0.74-0.16 vs 0.60-0.11). The diagnostic performance to detect overt hypothyroidism, isolated hypothyroxinemia, and (sub)clinical hyperthyroidism mainly varied between FT4 RI approaches, while the diagnostic performance to detect subclinical hypothyroidism varied between the applied TSH RI approaches.
Conclusion: Alternative approaches to define RIs for TSH and FT4 in pregnancy result in considerable overdiagnosis and underdiagnosis compared with population- and trimester-specific RIs. Additional strategies need to be explored to optimize identification of thyroid dysfunction during pregnancy.
Keywords: pregnancy; reference values; thyroid function tests; thyroid gland; thyrotropin; thyroxine.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Jansen TA, Korevaar TIM, Mulder TA, et al. . Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol. 2019;7(8):629‐637. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

