Desirability of outcome ranking for obstetrical trials: illustration and application to the ARRIVE trial
- PMID: 37741532
- PMCID: PMC10939984
- DOI: 10.1016/j.ajog.2023.09.016
Desirability of outcome ranking for obstetrical trials: illustration and application to the ARRIVE trial
Abstract
Background: In randomized trials, 1 primary outcome is typically chosen to evaluate the consequences of an intervention, whereas other important outcomes are relegated to secondary outcomes. This issue is amplified for many obstetrical trials in which an intervention may have consequences for both the pregnant person and the child. In contrast, desirability of outcome ranking, a paradigm shift for the design and analysis of clinical trials based on patient-centric evaluation, allows multiple outcomes-including from >1 individual-to be considered concurrently.
Objective: This study aimed to describe desirability of outcome ranking methodology tailored to obstetrical trials and to apply the methodology to maternal-perinatal paired (dyadic) outcomes in which both individuals may be affected by an intervention but may experience discordant outcomes (eg, an obstetrical intervention may improve perinatal but worsen maternal outcomes).
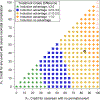
Study design: This secondary analysis applies the desirability of outcome ranking methodology to data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network ARRIVE trial. The original analysis found no substantial difference in the primary (perinatal composite) outcome, but a decreased risk of the secondary outcome of cesarean delivery with elective induction at 39 weeks. In the present desirability-of-outcome-ranking analysis, dyadic outcomes ranging from spontaneous vaginal delivery without severe neonatal complication (most desirable) to cesarean delivery with perinatal death (least desirable) were classified into 8 categories ranked by overall desirability by experienced investigators. Distributions of the desirability of outcome ranking were compared by estimating the probability of having a more desirable dyadic outcome with elective induction at 39 weeks of gestation than with expectant management. To account for various perspectives on these outcomes, a complementary analysis, called the partial credit strategy, was used to grade outcomes on a 100-point scale and estimate the difference in overall treatment scores between groups using a t test.
Results: All 6096 participants from the trial were included. The probability of a better dyadic outcome for a randomly selected patient who was randomized to elective induction was 53% (95% confidence interval, 51-54), implying that elective induction led to a better overall outcome for the dyad when taking multiple outcomes into account concurrently. Furthermore, the desirability-of-outcome-ranking probability of averting cesarean delivery with elective induction was 52% (95% confidence interval, 51-53), which was not at the expense of an operative vaginal delivery or a poorer outcome for the perinate (ie, survival with a severe neonatal complication or perinatal death). Randomization to elective induction was also advantageous in most of the partial credit score scenarios.
Conclusion: Desirability-of-outcome-ranking methodology is a useful tool for obstetrical trials because it provides a concurrent view of the effect of an intervention on multiple dyadic outcomes, potentially allowing for better translation of data for decision-making and person-centered care.
Keywords: desirability of outcome ranking; dyadic outcome; induction of labor.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Oxytocin regimen used for induction of labor and pregnancy outcomes.Am J Obstet Gynecol MFM. 2024 Dec;6(12):101508. doi: 10.1016/j.ajogmf.2024.101508. Epub 2024 Sep 30. Am J Obstet Gynecol MFM. 2024. PMID: 39357802 Clinical Trial.
-
Induction of labour at or beyond 37 weeks' gestation.Cochrane Database Syst Rev. 2020 Jul 15;7(7):CD004945. doi: 10.1002/14651858.CD004945.pub5. Cochrane Database Syst Rev. 2020. PMID: 32666584 Free PMC article.
-
Elective induction of labor at 39 weeks compared with expectant management: a meta-analysis of cohort studies.Am J Obstet Gynecol. 2019 Oct;221(4):304-310. doi: 10.1016/j.ajog.2019.02.046. Epub 2019 Feb 25. Am J Obstet Gynecol. 2019. PMID: 30817905
-
Maternal and newborn outcomes with elective induction of labor at term.Am J Obstet Gynecol. 2019 Mar;220(3):273.e1-273.e11. doi: 10.1016/j.ajog.2019.01.223. Epub 2019 Feb 17. Am J Obstet Gynecol. 2019. PMID: 30716284
-
An hypothetical external validation of the ARRIVE trial in a European academic hospital.J Matern Fetal Neonatal Med. 2022 Nov;35(22):4291-4298. doi: 10.1080/14767058.2020.1849108. Epub 2020 Nov 18. J Matern Fetal Neonatal Med. 2022. PMID: 33207972
Cited by
-
Integrating parent voices into research at the extremes of prematurity: what are we doing and where should we go?J Perinatol. 2025 Jun;45(6):699-704. doi: 10.1038/s41372-024-02165-1. Epub 2024 Nov 15. J Perinatol. 2025. PMID: 39548268 Free PMC article. Review.
-
Beneficial vs harmful effects of pharmacological treatment of patent ductus arteriosus: A Bayesian meta-analysis.Pediatr Res. 2025 Jan 15. doi: 10.1038/s41390-025-03820-9. Online ahead of print. Pediatr Res. 2025. PMID: 39815089
-
Perinatal mortality and other severe adverse outcomes following planned birth at 39 weeks versus expectant management in low-risk women: a population based cohort study.EClinicalMedicine. 2025 Jan 25;80:103076. doi: 10.1016/j.eclinm.2025.103076. eCollection 2025 Feb. EClinicalMedicine. 2025. PMID: 39927106 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- UG1 HD068258/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD068268/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- U10 HD068282/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- UG1 HD068268/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- U10 HD068258/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD068282/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources

