Monkeypox virus-infected individuals mount comparable humoral immune responses as Smallpox-vaccinated individuals
- PMID: 37741831
- PMCID: PMC10517934
- DOI: 10.1038/s41467-023-41587-x
Monkeypox virus-infected individuals mount comparable humoral immune responses as Smallpox-vaccinated individuals
Abstract
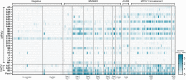
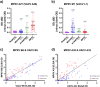
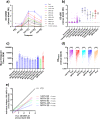
In early 2022, a cluster of monkeypox virus (MPXV) infection (mpox) cases were identified within the UK with no prior travel history to MPXV-endemic regions. Subsequently, case numbers exceeding 80,000 were reported worldwide, primarily affecting gay, bisexual, and other men who have sex with men (GBMSM). Public health agencies worldwide have offered the IMVANEX Smallpox vaccination to these individuals at high-risk to provide protection and limit the spread of MPXV. We have developed a comprehensive array of ELISAs to study poxvirus-induced antibodies, utilising 24 MPXV and 3 Vaccinia virus (VACV) recombinant antigens. Panels of serum samples from individuals with differing Smallpox-vaccine doses and those with prior MPXV infection were tested on these assays, where we observed that one dose of Smallpox vaccination induces a low number of antibodies to a limited number of MPXV antigens but increasing with further vaccination doses. MPXV infection induced similar antibody responses to diverse poxvirus antigens observed in Smallpox-vaccinated individuals. We identify MPXV A27 as a serological marker of MPXV-infection, whilst MPXV M1 (VACV L1) is likely IMVANEX-specific. Here, we demonstrate analogous humoral antigen recognition between both MPXV-infected or Smallpox-vaccinated individuals, with binding to diverse yet core set of poxvirus antigens, providing opportunities for future vaccine (e.g., mRNA) and therapeutic (e.g., mAbs) design.
© 2023. Crown.
Conflict of interest statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the UK Health Security Agency or the Centres for Disease Control and Prevention. The authors declare no competing interests.
Figures


References
-
- Shchelkunov SN. Emergence and reemergence of smallpox: the need for development of a new generation smallpox vaccine. Vaccine. 2011;29:D49–D53. - PubMed
-
- Malshetty VS, Jain R, Srinath T, Kurthkoti K, Varshney U. Synergistic effects of UdgB and Ung in mutation prevention and protection against commonly encountered DNA damaging agents in Mycobacterium smegmatis. Microbiology. 2010;156:940–949. - PubMed
-
- Priyamvada L, Satheshkumar PS. Variola and Monkeypox Viruses (Poxviridae) Encycl. Virol. 2021;2:868–874.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

