SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: a systematic review and meta-analysis
- PMID: 37741858
- PMCID: PMC10517929
- DOI: 10.1038/s41598-023-42989-z
SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: a systematic review and meta-analysis
Abstract
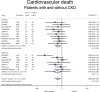
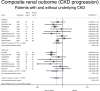
The effect of sodium-glucose co-transporter-2 (SGLT-2) inhibitors on cardiovascular and renal outcomes has not been systematically reviewed across baseline kidney function groups. We conducted a systematic review and meta-analysis of randomized control trials (RCTs) with SGLT-2 inhibitors in patients with and without CKD. We performed a PubMed/Medline search of randomized, placebo-controlled, event-driven outcome trials of SGLT-2 inhibitors versus active or placebo control in patients with and without diabetes from inception to November 2022. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 (PROSPERO registration CRD4202016054). The primary outcome was cardiovascular death. Secondary outcomes included hospitalization for heart failure, major adverse cardiovascular events, CKD progression, all-cause mortality, treatment discontinuation, and acute kidney injury (AKI). The relative risk (RR) was estimated using a random-effects model. Twelve RCTs were included in this meta-analysis (89,191 patients, including 38,949 with eGFR < 60 ml/min/1.73m2). Use of an SGLT-2 inhibitor in patients with CKD was associated with a lower incidence of cardiovascular death (RR 0.87; 95% CI 0.79-0.95) and of heart failure (RR 0.67; 95% CI 0.61-0.75), compared with placebo. Heart failure risk reduction with SGLT-2 inhibitors was larger among patients with CKD compared with patients without CKD (RR for the interaction 0.87, 95% CI 0.75-1.02, and p-value for interaction 0.08). SGLT-2 inhibitors were associated with a lower incidence of CKD progression among patients with pre-existing CKD: RR 0.77 (95% CI 0.68-0.88), compared with placebo. Among patients with CKD, a lower risk of AKI (RR 0.82; 95% CI 0.72-0.93) and treatment discontinuation was seen with SGLT-2 inhibitors compared with placebo. SGLT-2 inhibitors offer substantial protection against cardiovascular and renal outcomes in patients with CKD. These results strongly advocate in favor of using them in patients with CKD and keeping them as kidney function declines.
© 2023. Springer Nature Limited.
Conflict of interest statement
Dr. Mavrakanas received speaker honoraria from Daiichi Sankyo, BMS Canada, Janssen, Astra Zeneca, and Pfizer and has served on advisory boards for Boehringer Ingelheim, Bayer, GSK, and Servier outside the submitted work. He has also received an unrestricted research grant from Astra Zeneca. Dr. Tsoukas received speaker honoraria from NovoNordisk, Boehringer-Ingelheim, Janssen, Eli Lilly, and AstraZeneca outside the submitted work. Dr. Sharma received research and personal support from Roche Diagnostics, Boehringer-Ingelheim, Servier, Novartis, AstraZeneca, Janssen, Novo-Nordisk, BMS-Pfizer, and Takeda. The remaining authors have nothing to disclose.
Figures



References
-
- Solomon J, Festa MC, Chatzizisis YS, Samanta R, Suri RS, Mavrakanas TA. Sodium-glucose co-transporter 2 inhibitors in patients with chronic kidney disease. Pharmacol. Ther. 2022;10(242):108330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

