Phosphoflow cytometry to assess cytokine signaling pathways in peripheral immune cells: potential for inferring immune cell function and treatment response in patients with solid tumors
- PMID: 37741983
- PMCID: PMC10517546
- DOI: 10.1186/s13046-023-02802-1
Phosphoflow cytometry to assess cytokine signaling pathways in peripheral immune cells: potential for inferring immune cell function and treatment response in patients with solid tumors
Abstract
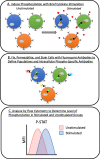
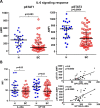
Tumor biopsy is often not available or difficult to obtain in patients with solid tumors. Investigation of the peripheral immune system allows for in-depth and dynamic profiling of patient immune response prior to and over the course of treatment and disease. Phosphoflow cytometry is a flow cytometry‒based method to detect levels of phosphorylated proteins in single cells. This method can be applied to peripheral immune cells to determine responsiveness of signaling pathways in specific immune subsets to cytokine stimulation, improving on simply defining numbers of populations of cells based on cell surface markers. Here, we review studies using phosphoflow cytometry to (a) investigate signaling pathways in cancer patients' peripheral immune cells compared with healthy donors, (b) compare immune cell function in peripheral immune cells with the tumor microenvironment, (c) determine the effects of agents on the immune system, and (d) predict cancer patient response to treatment and outcome. In addition, we explore the use and potential of phosphoflow cytometry in preclinical cancer models. We believe this review is the first to provide a comprehensive summary of how phosphoflow cytometry can be applied in the field of cancer immunology, and demonstrates that this approach holds promise in exploring the mechanisms of response or resistance to immunotherapy both prior to and during the course of treatment. Additionally, it can help identify potential therapeutic avenues that can restore normal immune cell function and improve cancer patient outcome.
Keywords: Clinical response; PBMC; Phosphoflow cytometry; Signaling; Solid tumors.
© 2023. Italian National Cancer Institute ‘Regina Elena’.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55(2):61–70. - PubMed
-
- Goldeck D, Low I, Shadan NB, Mustafah S, Pawelec G, Larbi A. Multi-parametric phospho-flow cytometry: a crucial tool for T lymphocyte signaling studies. Cytometry A. 2013;83(3):265–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

