Telomeric i-motifs and C-strands inhibit parallel G-quadruplex extension by telomerase
- PMID: 37742080
- PMCID: PMC10602923
- DOI: 10.1093/nar/gkad764
Telomeric i-motifs and C-strands inhibit parallel G-quadruplex extension by telomerase
Abstract
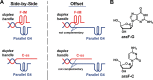
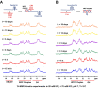
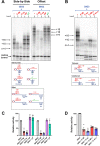
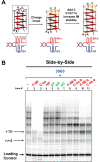
Telomeric C-rich repeated DNA sequences fold into tetrahelical i-motif structures in vitro at acidic pH. While studies have suggested that i-motifs may form in cells, little is known about their potential role in human telomere biology. In this study, we explore the effect of telomeric C-strands and i-motifs on the ability of human telomerase to extend G-rich substrates. To promote i-motif formation at neutral pH, we use telomeric sequences where the cytidines have been substituted with 2'-fluoroarabinocytidine. Using FRET-based studies, we show that the stabilized i-motifs resist hybridization to concomitant parallel G-quadruplexes, implying that both structures could exist simultaneously at telomeric termini. Moreover, through telomerase activity assays, we show that both unstructured telomeric C-strands and telomeric i-motifs can inhibit the activity and processivity of telomerase extension of parallel G-quadruplexes and linear telomeric DNA. The data suggest at least three modes of inhibition by C-strands and i-motifs: direct hybridization to the substrate DNA, hybridization to nascent product DNA resulting in early telomerase dissociation, and interference with the unique mechanism of telomerase unwinding and extension of a G-quadruplex. Overall, this study highlights a potential inhibitory role for the telomeric C-strand in telomere maintenance.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Fleming A.M., Ding Y., Rogers R.A., Zhu J., Zhu J., Burton A.D., Carlisle C.B., Burrows C.J.. 4n-1 Is a “sweet spot” in DNA i-motif folding of 2′-deoxycytidine homopolymers. J. Am. Chem. Soc. 2017; 139:4682–4689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

