Reconceptualization of the Erlangen Score for the Assessment of Dementia Risk: The ERlangen Score
- PMID: 37742651
- PMCID: PMC10657695
- DOI: 10.3233/JAD-230524
Reconceptualization of the Erlangen Score for the Assessment of Dementia Risk: The ERlangen Score
Abstract
Background: The established Erlangen Score (ES) for the interpretation of cerebrospinal fluid (CSF) biomarkers in the diagnostics of Alzheimer's disease (AD) uses markers of amyloidopathy and tauopathy, equally weighted to form an easy-interpretable ordinal scale. However, these biomarkers are not equally predictive for AD.
Objective: The higher weighting of the Aβ42/Aβ40 ratio, as a reconceptualized ERlangen Score (ERS), was tested for advantages in diagnostic performance.
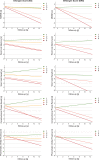
Methods: Non-demented subjects (N = 154) with a mean follow up of 5 years were assigned to a group ranging from 0 to 4 in ES or ERS. Psychometric trajectories and dementia risk were assessed.
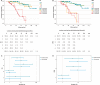
Results: The distribution of subjects between ES and ERS among the groups differed considerably, as grouping allocated 32 subjects to ES group 2, but only 2 to ERS group 2. The discriminative accuracy between the ES (AUC 73.2%, 95% CI [64.2, 82.2]) and ERS (AUC 72.0%, 95% CI [63.1, 81.0]) for dementia risk showed no significant difference. Without consideration of the Aβ42/Aβ40 ratio in ES grouping, the optimal cut-off of the ES shifted to ≥2.
Conclusions: The ERS showed advantages over the ES in test interpretation with comparable overall test performance, as fewer cases were allocated to the intermediate risk group. The established cut-off of ≥2 can be maintained for the ERS, whereas it must be adjusted for the ES when determining the Aβ42/Aβ40 ratio.
Keywords: Alzheimer’s disease; Erlangen Score; dementia risk; longitudinal study; neuropsychological trajectories.
Conflict of interest statement
PL received consultation and/or lecture honoraria from IBL International, Fujirebio Europe, AJ Roboscreen, Biogen, and Roche. PL is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. - PMC - PubMed
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert M-O, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, Souza LC de, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13, 614–629. - PubMed
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. - PMC - PubMed
-
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E (1995) Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 26, 231–245. - PubMed
-
- Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moller H-J, Davies P, Blennow K (2004) Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch Gen Psychiatry 61, 95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

