The Origin and Fate of Liver Myofibroblasts
- PMID: 37743012
- PMCID: PMC10665929
- DOI: 10.1016/j.jcmgh.2023.09.008
The Origin and Fate of Liver Myofibroblasts
Abstract
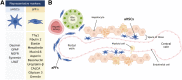
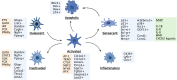
Liver fibrosis of different etiologies is a serious health problem worldwide. There is no effective therapy available for liver fibrosis except the removal of the underlying cause of injury or liver transplantation. Development of liver fibrosis is caused by fibrogenic myofibroblasts that are not present in the normal liver, but rather activate from liver resident mesenchymal cells in response to chronic toxic or cholestatic injury. Many studies indicate that liver fibrosis is reversible when the causative agent is removed. Regression of liver fibrosis is associated with the disappearance of activated myofibroblasts and resorption of the fibrous scar. In this review, we discuss the results of genetic tracing and cell fate mapping of hepatic stellate cells and portal fibroblasts, their specific characteristics, and potential phenotypes. We summarize research progress in the understanding of the molecular mechanisms underlying the development and reversibility of liver fibrosis, including activation, apoptosis, and inactivation of myofibroblasts.
Keywords: Hepatic Stellate Cells; Liver Fibrosis; Portal Fibroblasts.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Friedman S.L. Liver fibrosis - from bench to bedside. J Hepatol. 2003;38:38–53. - PubMed
-
- Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. - PubMed
-
- Sagnelli E., Macera M., Russo A., et al. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48:7–17. - PubMed
-
- Browning J.D., Szczepaniak L.S., Dobbins R., et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. - PubMed

