Optimal number of neoadjuvant chemotherapy cycles prior to interval debulking surgery in advanced epithelial ovarian cancer: a systematic review and meta-analysis of progression-free survival and overall survival
- PMID: 37743060
- PMCID: PMC10627748
- DOI: 10.3802/jgo.2023.34.e82
Optimal number of neoadjuvant chemotherapy cycles prior to interval debulking surgery in advanced epithelial ovarian cancer: a systematic review and meta-analysis of progression-free survival and overall survival
Abstract
Objective: Neoadjuvant chemotherapy (NACT) represents a treatment option in patients with advanced epithelial ovarian cancer (AEOC) who are not good candidates for primary debulking surgery. Usually, 3 cycles of chemotherapy before surgery have been considered the best option for patient survival, although quite often some patients receive more than 3 cycles. The aim of this systematic review and meta-analysis was to identify the optimal number of NACT cycles reporting better survival in AEOC patients.
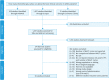
Methods: PubMed, Cochrane Library, and Scopus were searched for original articles that analyzed the relationship between the number of chemotherapy cycles and clinical outcomes in AEOC patients before interval debulking surgery (IDS). The main outcomes were progression-free survival (PFS) and overall survival (OS).
Results: A total of 22 studies comprising 7,005 patients diagnosed with AEOC were included in our analysis. In terms of survival, the reviewed studies dividing the patients in ≤3 NACT cycles vs. >3, showed a trend for a decrease in PFS and a significant reduction in OS with an increasing number of cycles, while a difference in both PFS and OS was revealed if early IDS included patients with 4 NACT cycles. These results should be interpreted with caution due to the complex characteristics of AEOC patients.
Conclusion: In conclusion, our review and meta-analysis revealed that there is not enough evidence to determine the optimal number of NACT treatments before surgery. Further research in the form of well-designed randomized controlled trials is necessary to address this issue.
Trial registration: PROSPERO Identifier: CRD42022334959.
Keywords: Cytoreductive Surgery; Neoadjuvant Chemotherapy; Ovarian Cancer; Prognosis; Survival.
© 2023. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


Similar articles
-
Comparing Laparotomy with Robot-assisted Interval Debulking Surgery for Patients with Advanced Epithelial Ovarian Cancer Receiving Neoadjuvant Chemotherapy.J Minim Invasive Gynecol. 2021 Jun;28(6):1237-1243. doi: 10.1016/j.jmig.2020.11.015. Epub 2020 Nov 26. J Minim Invasive Gynecol. 2021. PMID: 33248314
-
Primary debulking surgery versus primary neoadjuvant chemotherapy for high grade advanced stage ovarian cancer: comparison of survivals.Radiol Oncol. 2018 Sep 11;52(3):307-319. doi: 10.2478/raon-2018-0030. Radiol Oncol. 2018. PMID: 30210049 Free PMC article.
-
[Analysis of factors related to the prognostic benefit of neoadjuvant chemotherapy followed by interval debulking surgery in patients with advanced ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2021 Jun 25;56(6):385-392. doi: 10.3760/cma.j.cn112141-20201207-00871. Zhonghua Fu Chan Ke Za Zhi. 2021. PMID: 34154313 Chinese.
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2019 Oct 31;2019(10):CD005343. doi: 10.1002/14651858.CD005343.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 5;2:CD005343. doi: 10.1002/14651858.CD005343.pub5. PMID: 31684686 Free PMC article. Updated.
-
Survival outcome and perioperative complication related to neoadjuvant chemotherapy with carboplatin and paclitaxel for advanced ovarian cancer: A systematic review and meta-analysis.Eur J Surg Oncol. 2020 May;46(5):868-875. doi: 10.1016/j.ejso.2019.11.520. Epub 2019 Dec 4. Eur J Surg Oncol. 2020. PMID: 31818526 Free PMC article.
Cited by
-
Primary or Interval Debulking Surgery for Advanced Endometrial Cancer with Carcinosis: A Systematic Review and Individual Patient Data Meta-Analysis of Survival Outcomes.Cancers (Basel). 2025 Mar 19;17(6):1026. doi: 10.3390/cancers17061026. Cancers (Basel). 2025. PMID: 40149359 Free PMC article. Review.
-
Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update.J Clin Oncol. 2025 Mar;43(7):868-891. doi: 10.1200/JCO-24-02589. Epub 2025 Jan 22. J Clin Oncol. 2025. PMID: 39841949 Free PMC article.
-
Treatment of ovarian cancer: From the past to the new era (Review).Oncol Lett. 2025 Jun 3;30(2):384. doi: 10.3892/ol.2025.15130. eCollection 2025 Aug. Oncol Lett. 2025. PMID: 40535104 Free PMC article. Review.
References
-
- du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2005;16(Suppl 8):viii7–viiiviii12. - PubMed
-
- Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;29:728–760. - PubMed
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. - PubMed
-
- Bean J. EORTC trial 55971 compares treatment options for patients with stage IIIC or IV ovarian carcinoma [Internet] Brussels: EORTC; 2010. [cited 2022 Dec 16]. Available from: https://www.eortc.org/blog/2010/09/02/eortc-trial-55971-compares-treatme...

