Rnd3 suppresses endothelial cell pyroptosis in atherosclerosis through regulation of ubiquitination of TRAF6
- PMID: 37743632
- PMCID: PMC10518494
- DOI: 10.1002/ctm2.1406
Rnd3 suppresses endothelial cell pyroptosis in atherosclerosis through regulation of ubiquitination of TRAF6
Abstract
Background: As the main pathological basis for various cardiovascular and cerebrovascular diseases, atherosclerosis has become one of the leading causes of death and disability worldwide. Emerging evidence has suggested that Rho GTPase Rnd3 plays an indisputable role in cardiovascular diseases, although its function in atherosclerosis remains unclear. Here, we found a significant correlation between Rnd3 and pyroptosis of aortic endothelial cells (ECs).
Methods: ApoeKO mice were utilized as a model for atherosclerosis. Endothelium-specific transgenic mice were employed to disrupt the expression level of Rnd3 in vivo. Mechanistic investigation of the impact of Rnd3 on endothelial cell pyroptosis was carried out using liquid chromatography tandem mass spectrometry (LC-MS/MS), co-immunoprecipitation (Co-IP) assays, and molecular docking.
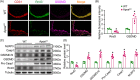
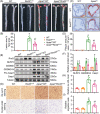
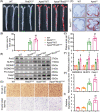
Results: Evidence from gain-of-function and loss-of-function studies denoted a protective role for Rnd3 against ECs pyroptosis. Downregulation of Rnd3 sensitized ECs to pyroptosis under oxidized low density lipoprotein (oxLDL) challenge and exacerbated atherosclerosis, while overexpression of Rnd3 effectively prevented these effects. LC-MS/MS, Co-IP assay, and molecular docking revealed that Rnd3 negatively regulated pyroptosis signaling by direct interaction with the ring finger domain of tumor necrosis factor receptor-associated factor 6 (TRAF6). This leads to the suppression of K63-linked TRAF6 ubiquitination and the promotion of K48-linked TRAF6 ubiquitination, inhibiting the activation of NF-κB and promoting the degradation of TRAF6. Moreover, TRAF6 knockdown countered Rnd3 knockout-evoked exacerbation of EC pyroptosis in vivo and vitro.
Conclusions: These findings establish a critical functional connection between Rnd3 and the TRAF6/NF-κB/NLRP3 signaling pathway in ECs, indicating the essential role of Rnd3 in preventing pyroptosis of ECs.
Keywords: Rho-GTPase; atherosclerosis; endothelial cell; pyroptosis; ubiquitination.
© 2023 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
